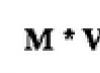Stomach enzymes, which appear as a result of the work of the gastrointestinal tract, play an important role in the digestion process. The digestive system is one of the main ones, since the functioning of the body as a whole depends on its functioning. Digestion is understood as a set of chemical and physical processes, as a result of the interaction of which various necessary compounds that enter the body with food are broken down into simpler compounds.
Basics of Human Digestion
The oral cavity is the starting point of the digestive process, and the large intestine is the final point. At the same time, digestion in its structure has two main components: mechanical and chemical processing of food that enters the body. At the initial point, a mechanical type of processing occurs, which includes grinding and grinding the food.
The gastrointestinal tract processes food through peristalsis, which promotes mixing. The chemical process of processing chyme includes salivation, during which carbohydrates are broken down, and food entering the body begins to be saturated with various vitamins. In the gastric cavity, slightly processed chyme is exposed to hydrochloric acid, which accelerates the process of breakdown of microelements. After this, the substances begin to interact with various enzymes that appear thanks to the work of the pancreas and other organs.
What are the digestive enzymes of the stomach?
 In the patient's stomach, protein particles and fats are mainly broken down. The main components of the breakdown of proteins and other particles are considered to be various enzymes, together with hydrochloric acid, produced by the mucous membrane. All these components together are called gastric juice. It is in the gastrointestinal tract that all microelements necessary for the body are digested and absorbed. At the same time, the enzymes necessary for digestion move into the intestines from the liver, salivary glands and pancreas.
In the patient's stomach, protein particles and fats are mainly broken down. The main components of the breakdown of proteins and other particles are considered to be various enzymes, together with hydrochloric acid, produced by the mucous membrane. All these components together are called gastric juice. It is in the gastrointestinal tract that all microelements necessary for the body are digested and absorbed. At the same time, the enzymes necessary for digestion move into the intestines from the liver, salivary glands and pancreas.
The upper layer of the intestine is covered with many secretory cells that secrete mucus, which protects vitamins, enzymes and layers deeper. The main role of mucus is to create conditions for easier movement of food into the intestinal zone. In addition, it performs a protective function, which is to reject chemical compounds. Thus, approximately 7 liters of digestive juices, which include digestive enzymes and mucus, can be produced per day.
There are a large number of factors that accelerate or slow down the secretory processes of enzymes. Any disruptions in the body lead to the fact that enzymes can be released in incorrect quantities, and this leads to a deterioration in the digestion process.
Types of enzymes and their description
Enzymes that promote the digestion process are secreted in all parts of the gastrointestinal tract. They significantly speed up and improve the processing of chyme and break down various compounds. But if their number changes, this may indicate the presence of diseases in the body. Enzymes can perform one or several functions. Depending on their location, several types are distinguished.
Enzymes produced in the mouth
- One of the enzymes produced in the oral cavity is ptyalin, which breaks down carbohydrates. Moreover, its activity remains in a slightly alkaline environment, at a temperature of about 38 degrees.
- The next type is the elements of amylase and maltase, which break down the disaccharides of maltose into glucose. They remain active under the same conditions as ptyalin. The enzyme can be found in the structure of blood, liver or saliva. Thanks to their work, various fruits quickly begin to be digested in the oral cavity, which then enter the stomach in a lighter form.
Enzymes produced in the gastric cavity

- The first proteolytic enzyme is pepsin, through which protein is broken down. Its initial form is pepsinogen, which is inactive due to the fact that it has an extra part. When it is affected by hydrochloric acid, this part begins to separate, which ultimately leads to the formation of pepsin, which has several types (for example, pepsin A, gastricsin, pepsin B). Pepsins dissociate in such a way that the proteins formed in the process can easily dissolve in water. After this, the processed masses move to the intestinal zone, where the digestive process is completed. Here, absolutely all proteolytic enzymes produced earlier are finally absorbed.
- Lipase is an enzyme that breaks down fat (lipids). But in adults this element is not as important as in childhood. Due to high temperature and peristalsis, compounds decompose into smaller elements, under the influence of which the effectiveness of enzymatic influence increases. This helps simplify the digestion of fatty compounds in the intestines.
- In the human stomach, it increases the activity of enzymes due to the production of hydrochloric acid, which is considered an inorganic element and plays one of the main roles in the digestion process. It promotes the destruction of proteins and activates the activity of the listed substances. At the same time, the acid perfectly disinfects the gastric zone, preventing the proliferation of bacteria, which in the future can lead to putrefaction of food masses.
What are the consequences of enzyme deficiency?
 Patients who abuse alcohol often experience enzyme deficiency.
Patients who abuse alcohol often experience enzyme deficiency.
Elements that help the digestion process may be contained in the body in quantities that deviate from the norm. Most often this is observed when the patient abuses alcoholic beverages, fatty, smoked and salty foods, or smokes. As a result, various diseases of the digestive tract develop that require immediate treatment.
First of all, the patient begins to experience heartburn, flatulence, and unpleasant belching. In this case, the last sign may not be taken into account if it had a one-time manifestation. In addition, there may be excessive production of various enzymes resulting from the action of the fungus. Its activity contributes to digestive problems, resulting in pathological belching. But often this begins in cases of taking antibiotics, due to which the microflora dies out and dysbiosis develops. To eliminate unpleasant symptoms, you need to normalize your diet by removing from it foods that increase the level of gas production.
How to properly treat the condition?
What treatments are there for the condition? This question is asked by many patients who have problems with the digestive tract. But every person must remember: only a doctor can tell you which medicine is more suitable, taking into account the individual properties of the body.
These can be various drugs that normalize the production of enzymes (for example, Mezim) and also restore the gastrointestinal environment (Lactiale, which enriches the gastrointestinal tract with beneficial flora). Any disease is always easier to prevent. To do this, you need to lead an active lifestyle, start watching the foods you eat, don’t abuse alcohol and don’t smoke.
Water activity and pH are the most important internal factors in determining a product's susceptibility to the growth of spoilage microorganisms. Parallel control of these parameters shows better results than their separate regulation. The effect of these two combined effects is described in detail within the framework of barrier technology for microbiological control, and is one of the most difficult parts of the determination of potentially hazardous products by the US Food and Drug Administration (FDA).
This article addresses how water activity and pH can be used together to improve microbiological control when using milder preservative technologies, which can lead to improved quality and texture of food products.
How water activity prevents the growth of microorganisms
Like any other organism, microorganisms require water to grow. They absorb water by moving it through the cell membrane. The mechanism of this movement depends on the water activity gradient - water moves from a high water activity environment outside the cell to a low water activity environment inside the cell.
A decrease in water activity outside the cell to a certain level causes osmotic stress: the cell can no longer absorb water and goes into a state of rest. The cell does not die - it simply loses its ability to reproduce. Different microorganisms cope with osmotic stress in different ways. Therefore, the growth limits for each microorganism are different. Some types of mold and yeast have adapted to tolerate very low levels of water activity.
Each microorganism has its own level of water activity at which bacterial reproduction stops. Accordingly, maintaining water activity below this level will prevent the microorganism from multiplying sufficiently to cause infection or disease.
Water activity indicators to limit the growth of microorganisms in the product
| Water activity | Bacteria | Mold | Yeast | Main Products |
| 0.97 | Clostridium botulinum E
Pseudomonas fluorescens |
fresh meat, fresh and canned vegetables and fruits | ||
| 0.95 | Escherichia coli
Clostridium perfringens Salmonella spp. Vibrio cholerae |
lightly salted bacon, cooked sausage, nasal spray, eye drops | ||
| 0.94 | Clostridium botulinum A, B
Vibrio parahaemolyticus |
Stachybotrys atra | ||
| 0.93 | Bacillus cereus | Rhizopus nigricans | some cheeses, ham, baked goods, sweetened condensed milk, oral suspensions, sunscreen lotions | |
| 0.92 | Listeria monocytogenes | |||
| 0.91 | Bacillus subtilis | |||
| 0.90 | Staphylococcus aureus
(anaerobic) |
Trichothecium roseum | Saccharomyces
cerevisiae |
|
| 0.88 | Candida | |||
| 0.87 | Staphylococcus aureus
(aerobic) |
|||
| 0.85 | Aspergillus clavatus | sweetened condensed milk, aged cheeses (such as cheddar), smoked sausage (such as salami), jerky, bacon, most concentrated fruit juices, chocolate syrup, fruit cake, fudge, cough syrup, oral numbing suspensions | ||
| 0.84 | Byssochlamys nivea | |||
| 0.83 | Penicillium expansum
Penicillium islandicum Penicillium viridicatum |
Deharymoces hansenii | ||
| 0.82 | Aspergillus fumigatus
Aspergillus parasiticus |
|||
| 0.81 | Penicillium Penicillium cyclopium
Penicillium patulum |
|||
| 0.80 | Saccharomyces bailii | |||
| 0.79 | Penicillium martensii | |||
| 0.78 | Aspergillus flavus | jam, marmalade, marzipan, glazed fruit, molasses, dried figs, highly salted fish | ||
| 0.77 | Aspergillus niger
Aspergillus ochraceous |
|||
| 0.75 | Aspergillus restrictus
Aspergillus candidus |
|||
| 0.71 | Eurotium chevalieri | |||
| 0.70 | Eurotium amstelodami | |||
| 0.62 | Saccharomyces rouxii | dried fruit, corn syrup, licorice, marshmallows, chewing gum, dry pet food | ||
| 0.61 | Monascus bisporus | |||
| 0.60 | No microbial proliferation | |||
| 0.50 | No microbial proliferation | caramel, toffee, honey, noodles, ointment for external use | ||
| 0.40 | No microbial proliferation | whole egg powder, cocoa, cough drops with liquid center | ||
| 0.30 | No microbial proliferation | crackers, flour snacks, baking mixes, vitamin tablets, suppositories | ||
| 0.20 | No microbial proliferation | lollipops, milk powder, baby formula |
Limiting the growth of microorganisms allows water activity to be used to ensure food safety. Therefore, water activity measurement can be used as a critical control point when planning a hazard analysis system (HACCP).
Opportunities for shared impact
The growth limits listed in the table above assume that other conditions (pH, temperature, etc.) are optimal for the growth of the microorganism. It turns out that if we take a lower pH value of the product and control the water activity, then the water activity indicator in this case may be higher than those indicated in the table.
What is pH
pH is a measure of the acidity or alkalinity of a solution. Values from 0 to 7 indicate acidity, and from 7 to 14 indicate alkalinity. Neutral distilled water has a pH value of 7. Foods are usually neutral or acidic.
pH limits microbial growth
Just as with water activity, there are pH limits at which microorganisms stop growing. The table below shows threshold values for different types of microbes.
pH values limiting the growth of certain types of bacteria
| Microorganism | Minimum value |
Optimal value |
Maximum value |
| Clostridium perfringens | 5.5 — 5.8 | 7.2 | 8.9 |
| Vibrio vulnificus | 5 | 7.8 | 10.2 |
| Racillus cereus | 4.9 | 6 — 7 | 8.8 |
| Campylobacter spp. | 4.9 | 6.5 — 7.5 | 9 |
| Shigella spp. | 4.9 | 9.3 | |
| Vibrio parahaemolyticus | 4.8 | 7.8 — 8.6 | 11 |
| Clostridium botulinum toxin | 4.6 | 8.5 | |
| Clostridium botulinum growth | 4.6 | 8.5 | |
| Staphylococcus aureus growth | 4 | 6 — 7 | 10 |
| Staphylococcus aureus toxin | 4.5 | 7 — 8 | 9.6 |
| Enterohemorrhagic Escherichia coli | 4.4 | 6 — 7 | 9 |
| Listeria monocytogenes | 4.39 | 7 | 9.4 |
| Salmonella spp. | 4.21 | 7 — 7.5 | 9.5 |
| Yersinia enterocolitica | 4.2 | 7.2 | 9.6 |
A pH-neutral environment is optimal for the growth of microorganisms, but growth is also possible in more acidic environments. Most microorganisms stop growing at pH 5.0; some can continue to grow at pH 4.6 or even 4.4. Historically, pH 4.6 was considered to be the lower limit for the growth of microorganisms, but it is known that some can continue to grow even at pH 4.2
Application of pH correction
Thus, lowering pH is an effective way to preserve food and prevent the spread of microbes, so pH measurement can be used as a critical control point in HACCP planning.
Also, some manufacturers vary the pH of the product to change its taste - by pickling or ripening. To do this, the product is subjected to an enzymatic reaction or exposure to an acid (such as vinegar) to stimulate the production of lactic acid. Many chemical reactions are pH dependent and can be stopped or controlled by adjusting the pH.
Combined influence of water activity and pH
The combination of barrier factors such as pH and water activity allows for more effective control of the spread of microorganisms. Moreover, the combined effect of these barriers is higher than that of each of them separately. This means that microbial growth can be effectively controlled at levels of water activity or pH that would be considered unsafe on their own. The table below shows combinations of these indicators that can be used to determine whether additional parameters for the safe storage of a product (temperature, storage time) need to be monitored.
This table is relevant for products that have been heat-treated before packaging. It should be remembered that reducing water activity and pH does not lead to the death of microorganisms, but only to preventing their reproduction to levels dangerous to humans. Heat treatment kills all microorganisms except sporogenous ones, so the product can be packaged at higher levels of water activity and pH - the corresponding values 0.92 and 4.6 can be considered safe.
| Water activity value | pH: not higher than 4.6 | pH: above 4.6 – 5.6 | pH: above 5.6 |
| not higher than 0.92 | no special temperature and time conditions are required | no special temperature and time conditions are required | |
| above 0.92 - 0.95 | no special temperature and time conditions are required | no special temperature and time conditions are required | |
| above 0.95 | no special temperature and time conditions are required | product quality control required | product quality control required |
The following table applies to products that have not been cooked or have been cooked but not packaged.
| Water activity value | pH: below 4.2 | pH: 4.2 – 4.6 | pH: above 4.6 – 5.0 | pH: above 5.0 |
| above 0.88 | no special temperature and time conditions are required | no special temperature and time conditions are required | no special temperature and time conditions are required | no special temperature and time conditions are required |
| above 0.88 - 0.90 | no special temperature and time conditions are required | no special temperature and time conditions are required | no special temperature and time conditions are required | product quality control required |
| above 0.90 - 0.92 | no special temperature and time conditions are required | no special temperature and time conditions are required | product quality control required | product quality control required |
| above 0.92 | no special temperature and time conditions are required | product quality control required | product quality control required | product quality control required |
Another table shows the water activity and pH of some popular foods.
Water Activity and pH of Common Foods
Canned strawberries have a very high water activity at a fairly low pH. The presence of citric acid causes a low pH, which helps prevent the growth of microorganisms when water activity is high. Mustard also has a very low pH and high water activity levels. The safety of these products is due to the low pH, not high water activity. Maple syrup is safe at a nearly neutral pH - it has a lot of sugar, which means the water activity will be low.
The graph shows that there is no direct relationship between water activity and pH. If an acid is added to a product to lower the pH, this will affect the water activity in some way, because acidic substances are usually polar and react primarily with water. But, of course, a decrease in pH will not directly lead to a decrease in water activity.
How to control water activity
The easiest way is to dry or bake (to do this correctly, you first need to understand the sorption isotherm - the absorption of moisture) Also, water activity can be controlled by adding hygroscopic substances such as salt, sugar, high fructose corn syrup, sorbitol or maltodextrin.
How to control pH
The most common way to lower pH is through fermentation. In this process, “good” bacteria produce lactic acid, which lowers the pH of the product and prevents the growth of other microorganisms. Pickled, salted and fermented products, as well as raw smoked sausage and olives are produced using this method. The pH can also be lowered by adding acid (acetic, lactic, citric) directly to the food, or by adding ingredients that are naturally acidic, such as tomatoes in spaghetti sauce.
Our company offers solutions for simple and fast
Digestion is a complex multi-stage physiological process, during which food (a source of energy and nutrients for the body) entering the digestive tract undergoes mechanical and chemical processing.
Features of the digestion process
Digestion of food includes mechanical (moistening and grinding) and chemical processing. The chemical process involves a series of successive stages of breaking down complex substances into simpler elements, which are then absorbed into the blood.
Types of coagulant curds and enzymes
There are three types of enzymes.
Chymosin produced by fermentation
The activation process occurs by a mono- or bimolecular reaction, depending on the enzyme and conditions. This indicates that in most cases at least 85% of the amino acids are required to be identical with immunochemical cross-reactions.The enzyme has mainly endopeptide activity and very little exopeptide activity, this is due to the fact that the active site is extensive and can contain seven amino acid residues. For this reason, it has complex specificity and the enzyme appears to be nonspecific. Some existing aspartic proteases have molecular variants containing more or less enzymatic compositions, the microheterogeneity being more or less expressed by the set of coagulant enzymes. Microheterogeneity causes glycolysis, phosphorylation, deamidation or partial proteolysis.
This occurs with the obligatory participation of enzymes that accelerate processes in the body. Catalysts are produced and are part of the juices they secrete. The formation of enzymes depends on what environment is established in the stomach, oral cavity and other parts of the digestive tract at one time or another.
Having passed the mouth, pharynx and esophagus, food enters the stomach in the form of a mixture of liquid and crushed by teeth. This mixture, under the influence of gastric juice, turns into a liquid and semi-liquid mass, which is thoroughly mixed due to the peristalsis of the walls. Next it enters the duodenum, where it is further processed by enzymes.
Specific molecular aspects
It is characterized by high specificity of milk coagulation and, as a rule, low proteolytic activity. Quimogen, also called prochymosin, is converted into an active enzyme by acid treatment. This occurs through the pseudochymosin intermediate at pH 2, where the rate of activation is rapid, which converts to chymosin at high pH. They are characterized by a high degree of proteolytic activity and resistance to heat treatment. These enzymes are homologous but have different specificities. . Digestion of food occurs through a reaction called hydrolysis, which involves the breakdown of certain substances involving water molecules.
The nature of the food determines what kind of environment will be established in the mouth and stomach. Normally, the oral cavity has a slightly alkaline environment. Fruits and juices cause a decrease in the pH of the oral fluid (3.0) and the formation of an acidic environment. Products containing ammonium and urea (menthol, cheese, nuts) can cause the saliva reaction to become alkaline (pH 8.0).
Structure of the stomach
The stomach is a hollow organ in which food is stored, partially digested and absorbed. The organ is located in the upper half of the abdominal cavity. If you draw a vertical line through the navel and chest, then approximately 3/4 of the stomach will be to the left of it. In an adult, the stomach volume is on average 2-3 liters. When consuming a large amount of food, it increases, and if a person is starving, it decreases.
These hydrolysis reactions are catalyzed by enzymes commonly called hydrolytic enzymes. Digestive enzymes are biological catalysts released in the organs of the digestive system that promote chemical reactions that reduce molecules, smaller organic compounds, present in foods, allowing them to be absorbed and used by the body.
Digestive enzymes are named according to the substrate on which they act, whether carbohydrates, lipids or proteins. Protease carbohydrase Lipase Nuclease Maltase Amylase. . Enzymes are very large and complex protein molecules that act as catalysts in biochemical reactions. They act on starch by releasing various products, including dextrins and gradually small polymers consisting of glucose units. Produced in saliva and the pancreas, amylase is also produced by various fungi, bacteria and vegetables.
The shape of the stomach can change in accordance with its filling with food and gases, as well as depending on the condition of neighboring organs: pancreas, liver, intestines. The shape of the stomach is also influenced by the tone of its walls.
The stomach is an extended part of the digestive tract. At the entrance there is a sphincter (pyloric valve) that allows food to pass from the esophagus into the stomach in portions. The part adjacent to the entrance to the esophagus is called the cardiac part. To the left of it is the fundus of the stomach. The middle part is called the “body of the stomach”.
Amylases are divided into two groups: endoamylases and exoamylases. Endoamylases catalyze random hydrolysis in the starch molecule. Exoamylases exclusively hydrolyze -1,4 glycosidic linkages such as α-amylase or both α-1,4 and α-1,6 linkages such as amyloglucosidase and glycosidase. Amylase, like all other enzymes, acts as a catalyst, meaning it is not altered by the reaction but facilitates it, reducing the amount of energy required to achieve it. Amylase digests starches by catalyzing hydrolysis, which is the destruction by the addition of one molecule of water.
Between the antrum (end) of the organ and the duodenum there is another pylorus. Its opening and closing is controlled by chemical stimuli released from the small intestine.
Features of the structure of the stomach wall
The wall of the stomach is lined with three layers. The inner layer is the mucous membrane. It forms folds, and its entire surface is covered with glands (about 35 million in total), which secrete gastric juice and digestive enzymes intended for chemical processing of food. The activity of these glands determines what environment in the stomach - alkaline or acidic - will be established in a certain period.
Thus, starch plus water is formed in maltose. Other enzymes then break down the maltose into glucose, which is absorbed through the walls of the small intestine and, once taken into the liver, used as energy. In addition to the catalytic breakdown of starch molecules, fungal alpha-amylase is a multienzyme capable of performing more than 30 enzymatic functions, including the breakdown of fat and protein molecules. It is also capable of converting 450 times its own weight in starch into maltose. -Amylase catalyzes the hydrolysis of fats, converting them into glycerol and fatty acids, proteins into proteoses and starch derivatives into dextrin and simpler sugars.

The submucosa has a rather thick structure, penetrated by nerves and vessels.
The third layer is a powerful membrane, which consists of smooth muscle fibers necessary for processing and pushing food.
The outside of the stomach is covered with a dense membrane - the peritoneum.
It has an activity pH close to 7. Indications:? -Amylase accelerates and facilitates the digestion of starch, fats and proteins. Thus, it can increase the body's utilization of food and be used to treat pancreatic secretion deficiency and chronic pancreatic inflammation, among other benefits.
Contraindications: Should not be administered to patients with known hypersensitivity to the fungal enzyme. Adverse reactions: the possibility of allergic reactions in persons with hypersensitivity to the fungal enzyme. Lipases can be of plant, porcine or microbial origin, and the latter has a significant advantage. Helpful when production deficiency occurs in the pancreas, lipase is an enzyme whose supplementation may be beneficial in cases of indigestion, celiac disease, cystic fibrosis and Crohn's disease.
Gastric juice: composition and features
The main role at the stage of digestion is played by gastric juice. The glands of the stomach are varied in their structure, but the main role in the formation of gastric fluid is played by cells that secrete pepsinogen, hydrochloric acid and mucoid substances (mucus).

Lipase is responsible for the breakdown and absorption of fats in the intestines. An enzyme essential for the absorption and digestion of nutrients in the intestines, responsible for the breakdown of lipids, especially triglycerides, lipase allows the body to absorb food more easily by maintaining nutrients at appropriate levels. In the human body, lipase is produced mainly by the pancreas, but is also secreted by the oral cavity and stomach. Most people produce sufficient amounts of pancreatic lipase.
The use of lipase supplements may be advisable in cases of chronic indigestion. In a study of 18 people, supplements containing lipase and other pancreatic enzymes were shown to reduce stomach printing, tearing, gas and discomfort after eating a high-fat meal. Because some of these symptoms are associated with irritable bowel syndrome, some people with this condition may experience improvement with the use of pancreatic enzymes.
Digestive juice is a colorless, odorless liquid and determines what kind of environment should be in the stomach. It has a pronounced acidic reaction. When conducting a study to detect pathologies, it is easy for a specialist to determine what kind of environment exists in an empty (fasting) stomach. It is taken into account that normally the acidity of juice on an empty stomach is relatively low, but when secretion is stimulated it increases significantly.
Research suggests that lipase may be beneficial in cases of celiac disease, a condition in which gluten from food causes damage to the intestinal tract. Symptoms include abdominal pain, weight loss and fatigue. In a study of 40 children with celiac disease, those who received pancreatic therapy showed a slight increase in weight compared to the placebo group. People with pancreatic insufficiency and cystic fibrosis often need lipase and other enzyme supplements. People with celiac disease, Crohn's disease, or digestive disorders may be deficient in pancreatic enzymes, including lipase.
A person who adheres to a normal diet produces 1.5-2.5 liters of gastric fluid during the day. The main process occurring in the stomach is the initial breakdown of proteins. Since gastric juice affects the secretion of catalysts for the digestion process, it becomes clear in what environment the stomach enzymes are active - in an acidic environment.
Indications: In cases of pancreatic enzyme deficiency, dyspepsia, cystic fibrosis and celiac disease, Crohn's disease. Contraindications: there are no references in reference books. Adverse Reactions: There are no reports of side effects using the dosage suggested above.
Precautions: Lipase should not be taken concomitantly with betaine hydrochloride or hydrochloric acid, which may destroy the enzyme. Interactions: Talk to your doctor if the patient is taking orlistat, as it interferes with the activity of lipase supplements, blocking their ability to break down fats.
Enzymes produced by glands of the gastric mucosa
Pepsin is the most important enzyme in digestive juice, involved in the breakdown of proteins. It is produced under the influence of hydrochloric acid from its predecessor, pepsinogen. The action of pepsin is about 95% of the splitting juice. Factual examples show how high its activity is: 1 g of this substance is enough to digest 50 kg of egg white and curdle 100,000 liters of milk in two hours.
It is an enzyme secreted by the pancreas that is involved in the degradation of proteins resulting from the action of gastric pepsin. The protease is secreted as a proenzyme and is activated by intestinal juice. It is administered along with other pancreatic amylases and propancin lipases when there is a decrease in pancreatic secretion.
Proteases are enzymes that break down peptide bonds between amino acids in proteins. This process is called proteolytic cleavage, a common mechanism for activating or inactivating enzymes primarily involved in digestion and blood clotting.
Mucin (stomach mucus) is a complex complex of protein substances. It covers the entire surface of the gastric mucosa and protects it from both mechanical damage and self-digestion, since it can weaken the effect of hydrochloric acid, in other words, neutralize it.
Lipase is also present in the stomach - Gastric lipase is inactive and mainly affects milk fats.
Proteases occur naturally in all organisms and represent 1-5% of their genetic content. These enzymes are involved in a wide range of metabolic reactions, from simple digestion of food proteins to highly regulated cascades. Proteases are found in a variety of microorganisms such as viruses, bacteria, protozoa, yeast and fungi. The inability of plant and animal proteases to meet the global demand for enzymes has led to increasing interest in proteases of microbial origin.
Microorganisms are an excellent source of proteases due to their high biochemical diversity and ease of genetic manipulation. Numerous proteinases are produced by individual microorganisms, depending on the species, or even by different strains of the same species. Different proteinases can also be produced by the same strain by changing culture conditions.
Another substance that deserves mention is Castle's intrinsic factor, which promotes the absorption of vitamin B12. Let us remind you that vitamin B 12 is necessary for the transport of hemoglobin in the blood.
The role of hydrochloric acid in digestion
Hydrochloric acid activates enzymes in gastric juice and promotes the digestion of proteins, as it causes them to swell and loosen. In addition, it kills bacteria that enter the body with food. Hydrochloric acid is released in small doses, regardless of the environment in the stomach, whether there is food in it or whether it is empty.
Dosage: The dose varies from 600 units to 500 units. Contraindications: Should not be administered to patients with known hypersensitivity to the bacterial enzyme. Side effects: possibility of allergic reactions in persons with hypersensitivity to the bacterial enzyme.
Take 1 to 2 capsules with each meal. Pepsinogen is an inactive form of the enzyme. This precursor is secreted by the gastric mucosa and must be treated with hydrochloric acid to be active. About 1% of pepsinogen can enter the bloodstream and may be a useful indicator of gastric disease. In particular, its values are taken into account with the purpose.
But its secretion depends on the time of day: it has been established that the minimum level of gastric secretion is observed between 7 and 11 am, and the maximum at night. When food enters the stomach, acid secretion is stimulated due to increased activity of the vagus nerve, distension of the stomach and the chemical effect of food components on the mucous membrane.
Pepsinogen and pepsin: biological role and protein digestion
Monitor the health and functionality of the gastric mucosa; Assess the risk of developing gastritis; Determine the proportion of those affected as a result of certain pathological conditions. Pepsin is secreted as a zymogen, that is, in an inactive form that acquires functional capacity only after a precise structural change. Specifically, hydrochloric acid secreted by the parietal cells of the stomach converts pepsinogen, its precursor to pepsin, through a proteolytic cut, resulting in the removal of about forty amino acids.
What environment in the stomach is considered standard, norm and deviations
When talking about the environment in the stomach of a healthy person, it should be taken into account that different parts of the organ have different acidity values. So, the highest value is 0.86 pH, and the minimum is 8.3. The standard indicator of acidity in the body of the stomach on an empty stomach is 1.5-2.0; on the surface of the inner mucous layer the pH is 1.5-2.0, and in the depths of this layer - 7.0; in the final part of the stomach varies from 1.3 to 7.4.

Stomach diseases develop as a result of an imbalance of acid production and neiolysis and directly depend on the environment in the stomach. It is important that the pH values are always normal.
Prolonged hypersecretion of hydrochloric acid or inadequate acid neutralization leads to an increase in acidity in the stomach. In this case, acid-dependent pathologies develop.
Low acidity is characteristic of (gastroduodenitis) and cancer. The indicator for gastritis with low acidity is 5.0 pH or more. Diseases mainly develop with atrophy of the cells of the gastric mucosa or their dysfunction.
Gastritis with severe secretory insufficiency
The pathology occurs in mature and elderly patients. Most often, it is secondary, that is, it develops against the background of another disease that precedes it (for example, a benign stomach ulcer) and is the result of the environment in the stomach - alkaline, in this case.
The development and course of the disease is characterized by the absence of seasonality and a clear periodicity of exacerbations, that is, the time of their occurrence and duration are unpredictable.

Symptoms of secretory insufficiency
- Constant belching with a rotten taste.
- Nausea and vomiting during exacerbation.
- Anorexia (lack of appetite).
- Feeling of heaviness in the epigastric region.
- Alternating diarrhea and constipation.
- Flatulence, rumbling and transfusions in the stomach.
- Dumping syndrome: a feeling of dizziness after eating carbohydrate foods, which occurs due to the rapid entry of chyme from the stomach into the duodenum, with a decrease in gastric activity.
- Weight loss (weight loss is up to several kilograms).

Gastrogenic diarrhea can be caused by:
- poorly digested food entering the stomach;
- a sharp imbalance in the process of fiber digestion;
- accelerated gastric emptying in case of disruption of the closing function of the sphincter;
- violation of bactericidal function;
- pathologies of the pancreas.
Gastritis with normal or increased secretory function
This disease is more common in young people. It is of a primary nature, that is, the first symptoms appear unexpectedly for the patient, since before that he did not feel any pronounced discomfort and subjectively considered himself healthy. The disease occurs with alternating exacerbations and respites, without a pronounced seasonality. To accurately determine the diagnosis, you need to consult a doctor so that he can prescribe an examination, including an instrumental one.
In the acute phase, pain and dyspeptic syndromes predominate. Pain, as a rule, is clearly related to the environment in the human stomach at the time of eating. Pain occurs almost immediately after eating. Late fasting pain (some time after eating) is less common; a combination of both is possible.
Symptoms of increased secretory function
- The pain is usually moderate, sometimes accompanied by pressure and heaviness in the epigastric region.
- Late pain is intense.
- Dyspeptic syndrome is manifested by belching “sour” air, an unpleasant taste in the mouth, disturbances of taste, nausea, which relieves pain by vomiting.
- Patients experience heartburn, sometimes painful.
- Intestinal dyspepsia syndrome is manifested by constipation or diarrhea.
- Typically characterized by aggressiveness, mood swings, insomnia and fatigue.
E) gastrogenic insufficiency during gastrectomy, gastrectomy, atrophic gastritis.
2. Violation of parietal digestion due to deficiency of disaccharidases (congenital, acquired lactase or other disaccharidase deficiency), with disruption of the intracellular transport of food components as a result of the death of enterocytes (Crohn's disease, celiac enteropathy, sarcoidosis, radiation, ischemic and other enteritis).
3. Impaired lymph outflow from the intestines - obstruction of the lymphatic ducts with lymphangectasia, lymphoma, intestinal tuberculosis, carcinoid.
4. Combined disorders in diabetes mellitus, giardiasis, hyperthyroidism, hypogammaglobulinemia, amyloidosis, AIDS, sepsis.
All of the conditions listed above are, to one degree or another, indications for enzyme therapy.
Despite the variety of causes that cause digestive disorders, the most severe disorders are caused by diseases of the pancreas, which are accompanied by exocrine insufficiency. It occurs in diseases of the pancreas combined with insufficiency of its exocrine function (chronic pancreatitis, pancreatic fibrosis, etc.). Exocrine pancreatic insufficiency remains one of the most pressing problems in modern medicine. Every year in Russia, more than 500 thousand people go to medical institutions due to various pathologies of the pancreas, accompanied by exocrine insufficiency. In addition, even minor deviations in the chemical structure of food lead to the development of exocrine pancreatic insufficiency. In chronic pancreatitis, exocrine pancreatic insufficiency develops in later stages of the disease due to the progressive loss of functionally active parenchyma of the organ and its atrophy. In this case, clinical signs of maldigestion with loss of body weight come to the fore; systemic complications (immunodeficiency, infectious complications, neurological disorders, etc.) may also develop. In some cases, patients with chronic pancreatitis are not bothered by the pain symptom and the disease manifests itself as exocrine and/or endocrine insufficiency. A long-term history of chronic pancreatitis significantly increases the risk of developing pancreatic cancer. To date, it has been established that the main cause of the development of chronic pancreatitis with exocrine insufficiency is toxic-metabolic effects on the pancreas. In developed countries, alcohol abuse is the main cause of the development of chronic pancreatitis, especially in combination with a high protein and fat content in the drinkers' diet. In 55–80% of patients with chronic pancretitis with exocrine pancreatic insufficiency, the etiology of the disease is determined by alcohol. There is also evidence indicating a genetic predisposition to the development of chronic pancreatitis. In addition, cigarette smoking has recently been implicated in the development of chronic pancreatitis. Clinical signs of exocrine pancreatic insufficiency include flatulence, steatorrhea, nausea, weight loss, muscle atrophy, and fat-soluble vitamin deficiency. The symptom of abdominal pain with exocrine pancreatic insufficiency can be caused not only by concomitant pancreatitis, but also by overstretching of the intestinal wall due to excessive accumulation of gases and accelerated passage of feces. According to some authors, the pain symptom in exocrine pancreatic insufficiency may be due to the fact that reduced secretion of pancreatic enzymes in exocrine insufficiency leads to hyperstimulation of the pancreas by high levels of cholecystokinin in the blood plasma and, consequently, to abdominal pain syndrome. To diagnose exocrine insufficiency, laboratory and instrumental research methods are also used. Coprological research has not lost its relevance to this day and is an accessible informative method for determining the presence of exocrine pancreatic insufficiency. With functional deficiency, polyfecal matter appears, feces acquire a grayish tint, have a “greasy” appearance, a fetid, putrid odor, steatorrhea, creatorrhea, and rarely amilorrhea appear. Scatological examination is not always informative in case of mild disorders of exocrine function. Determining the content of elastase-1 in feces is one of the modern methods for assessing the severity of exocrine pancreatic insufficiency, since pancreatic elastase does not change its structure as it passes through the gastrointestinal tract. Also indispensable methods for diagnosing the cause that led to the development of exocrine pancreatic insufficiency are ultrasound examination of the pancreas, computed tomography, etc.
Therapy for digestive dysfunction is based on the use of enzyme preparations, the choice of which should be made taking into account the type, severity, reversibility of pathological changes and motor disorders of the gastrointestinal tract. Typically, enzyme preparations are multicomponent drugs, the basis of which is a complex of enzymes of animal, plant or fungal origin in pure form or in combination with auxiliary components (bile acids, amino acids, hemicellulase, simethicone, adsorbents, etc.).
In clinical practice, the choice and dosage of enzyme preparations is determined by the following main factors:
- composition and quantity of active digestive enzymes that ensure the breakdown of nutrients;
- release form of the drug: ensuring the resistance of enzymes to the action of hydrochloric acid; providing rapid release of enzymes in the duodenum; ensuring the release of enzymes in the range of 5–7 units. pH;
- well tolerated and no side effects;
- long shelf life.
Let's take a closer look at the drug Unienzyme with MPS with its unique complex enzyme composition (Table 1).
substances, lead active life thanks to:
a) omnivorous;
b) development with metamorphosis;
c) eating only protein-rich animal foods;
d) the ability to stay under water for a long time.
22. Breathing in amphibians is carried out:
a) through the gills;
b) through the lungs;
c) through the skin;
d) all of the above methods.
23. The tibia should be attributed to the level of organization of living things:
a) cellular;
b) tissue;
c) organ;
d) systemic.
The figure shows a fragment of a typical
Electrocardiogram (ECG) of a person obtained
At the second standard lead.
The T–P interval reflects the following process in
heart:
a) atrial stimulation;
b) restoration of the state of the ventricular myocardium
after reduction;
c) spread of excitation through the ventricles;
d) rest period - diastole.
25. Optimal environment for high activity of gastric enzymes:
a) alkaline;
b) neutral;
c) sour;
a) thoroughly rinse open wounds, remove dead tissue and consult a doctor;
b) place your hand in cold water or cover it with pieces of ice as soon as possible;
c) rub the limb until it turns red and apply a tight bandage;
d) bandage the burned limb tightly and consult a doctor.
Lymph is carried directly from tissues and organs through lymphatic vessels
a) arterial bed of the systemic circulation;
b) venous bed of the systemic circulation;
c) arterial bed of the pulmonary circulation;
d) venous bed of the pulmonary circulation.
28. Blood loses the maximum amount of oxygen when passing through:
a) lungs;
b) one of the veins of the arm;
c) capillaries in one of the muscles;
d) right atrium and right ventricle.
29. The nerve that rotates the eyeball in humans:
a) trigeminal;
b) block;
c) visual;
d) facial.
30. The volume of air that can be inhaled after a quiet exhalation is called:
a) expiratory reserve volume;
b) inspiratory reserve volume;
c) tidal volume;
d) residual volume.
The figure shows
Reconstruction of the external appearance and
Remains of primitive culture
One of the ancestors of modern
Human. This representative
should be classified as: 
a) human predecessors;
b) ancient people;
c) ancient people;
d) fossil people of modern times
anatomical type.
32. The adrenal cortex produces the hormone:
a) adrenaline;
b) thyroxine;
c) cortisone;
d) glucagon.
33. An extra link in a single trophic chain is:
a) earthworm;
b) bluegrass;
In natural communities, the role of 2nd order consumers is, as a rule,
can play:
a) bleak, warbler, roe deer, ground beetle;
b) nutcracker, quick lizard, starfish, hare;
c) duck, dog, spider, starling;
d) frog, vine snail, cat, buzzard.
Any study of the properties of enzymes, any application of them in practical activities - in medicine and in the national economy - is always associated with the need to know at what speed the enzymatic reaction proceeds. In order to understand and correctly evaluate the results of determining enzymatic activity, you need to clearly imagine on what factors the reaction rate depends and what conditions influence it. There are many such conditions. First of all, this is the ratio of the concentration of the reacting substances themselves: enzyme and substrate. Further, these are all sorts of features of the environment in which the reaction takes place: temperature, acidity, the presence of salts or other impurities that can both accelerate and slow down the enzymatic process, and so on.
The action of enzymes depends on a number of factors, primarily on temperature and the reaction of the environment (pH). The optimal temperature at which enzyme activity is highest is usually in the range of 37 – 50˚C. At lower temperatures, the rate of enzymatic reactions decreases, and at temperatures close to 0˚C it almost completely stops. As the temperature increases, the speed also decreases and finally stops completely. The decrease in enzyme intensity with increasing temperature is mainly due to the destruction of the protein included in the enzyme. Since proteins denature in the dry state much more slowly than when hydrated (in the form of a protein gel or solution), inactivation of enzymes in the dry state occurs much more slowly than in the presence of moisture. Therefore, dry bacterial spores or dry seeds can withstand heating to much higher temperatures than more moist seeds and spores.
For most currently known enzymes, an optimum pH has been determined at which they have maximum activity. This value is an important criterion for the characteristics of the enzyme. Sometimes this property of enzymes is used for their preparative separation. The presence of an optimum pH can be explained by the fact that enzymes are polyelectrolytes and their charge depends on the pH value. Sometimes accompanying substances can change the pH optimum, such as buffer solutions. In some cases, depending on the substrates, enzymes with weakly expressed specificity have several optima.
An important factor on which the action of enzymes depends, as Sørensen first established, is the active reaction of the environment - pH. Individual enzymes differ in the optimal pH value for their action. For example, pepsin contained in gastric juice is most active in a strongly acidic environment (pH 1 – 2); trypsin - a proteolytic enzyme secreted by the pancreas, has an optimum action in a slightly alkaline environment (pH 8 - 9); papain, an enzyme of plant origin, works optimally in a slightly acidic environment (pH 5 – 6).
It follows that the value (PH optimum) is a very sensitive sign for this enzyme. It depends on the nature of the substrate and the composition of the buffer solution and therefore is not a true constant. It is also necessary to keep in mind the properties of enzymes as protein bodies capable of acid-base denaturation. Acid-base denaturation can lead to irreversible changes in the structure of the enzyme with the loss of its catalytic properties.
The rate of any enzymatic process depends largely on the concentration of both substrate and enzyme. Typically, the reaction rate is directly proportional to the amount of enzyme, provided that the substrate content is within the optimum range or slightly higher. At a constant amount of enzyme, the rate increases with increasing substrate concentration. This reaction is subject to the law of mass action and is considered in the light of the Michaelis-Menton theory, that is,
V=K(F) ,
V - reaction speed
K - rate constant
F - enzyme concentration.
The presence of certain ions in the reaction medium can activate the formation of the active substrate of the enzyme complex, in which case the rate of the enzymatic reaction will increase. Such substances are called activators. In this case, substances that catalyze enzymatic reactions do not directly participate in them. The activity of some enzymes is significantly affected by the concentration of salts in the system, while other enzymes are not sensitive to the presence of ions. However, some ions are absolutely necessary for the normal functioning of some enzymes. Ions are known that inhibit the activity of some enzymes and are activators for others. Specific activators include metal cations: Na + , K + , Rb + , Cs + , Mg2 + , Ca2 + , Zn2 + , Cd2 + , Cr2 + , Cu2 + , Mn2 + , Co2 + , Ni2 + , Al3 + . It is also known that Fe2 + , Rb + , Cs + cations act as activators only in the presence of Mg; in other cases, these cations are not activators. In most cases, one or two ions can activate a particular enzyme. For example, Mg2 + - a common activator for many enzymes, acting on phosphorimated substrates, in almost all cases can be replaced by Mn2 +, although other metals cannot replace it. It should be noted that alkaline earth metals generally compete with each other, in particular, Ca2 + suppresses the activity of many enzymes activated by Mg2 + and Zn2 +. The reason for this is still unclear. The mechanism of influence of metal ions - activators can be different. First of all, the metal may be a component of the active site of the enzyme. But it can act as a connecting bridge between the enzyme and the substrate, holding the substrate at the active site of the enzyme. There is evidence that metal ions are capable of binding an organic compound to proteins and, finally, one of the possible mechanisms of action of metals as activators is a change in the equilibrium constant of the enzymatic reaction. It has been proven that anions also affect the activity of a number of enzymes. For example, the influence of CI on the activity of A-amylase of animal origin is very great.
The action of enzymes also depends on the presence of specific activators or inhibitors. Thus, the pancreatic enzyme enterokinase converts inactive trypsinogen into active trypsin. Such inactive enzymes contained in cells and in the secretions of various glands are called proenzymes. An enzyme can be competitive or non-competitive. In competitive inhibition, the inhibitor and substrate compete with each other, trying to displace each other from the enzyme-substrate complex. The effect of a competitive inhibitor is removed by high concentrations of the substrate, while the effect of a non-competitive inhibitor remains under these conditions. The effect of specific activators and inhibitors on the enzyme is of great importance for regulating enzymatic processes in the body.
Along with the existence of enzyme activators, a number of substances are known whose presence inhibits the catalytic action of enzymes or completely inactivates it. Such substances are usually called inhibitors. Inhibitors are substances that act in a certain chemical way on enzymes and, according to the nature of their action, can be divided into reversible and irreversible inhibitors. Reversible inhibition is characterized by an equilibrium between the enzyme and the inhibitor with a certain equilibrium constant. A system of this type is characterized by a certain degree of inhibition, depending on the concentration of the inhibitor, and the inhibition is achieved quickly and is then independent of time. When the inhibitor is removed by dialysis, enzyme activity is restored. Irreversible inhibition is primarily expressed in the fact that dialysis does not help restore enzyme activity. And in contrast to reversible inhibition, it increases with time, so that complete inhibition of the catalytic activity of the enzyme can occur at a very low concentration of inhibitor. In this case, the effectiveness of the inhibitor does not depend on the equilibrium constant, but on the rate constant, which determines the proportion of the enzyme that is inhibited in this case.