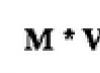Structural formula
True, empirical, or gross formula: CO2
Chemical composition of carbon dioxide
Molecular weight: 44.009
Carbon dioxide (carbon dioxide, carbon dioxide, carbon (IV) oxide, carbonic anhydride) is a colorless gas (under normal conditions), odorless, with a chemical formula CO2. Density under normal conditions is 1.98 kg/m³ (heavier than air). At atmospheric pressure, carbon dioxide does not exist in a liquid state, changing directly from a solid to a gaseous state. Solid carbon dioxide is called dry ice. At elevated pressure and normal temperatures, carbon dioxide turns into liquid, which is used for its storage. The concentration of carbon dioxide in the Earth's atmosphere averages 0.04%. Carbon dioxide easily transmits ultraviolet rays and rays of the visible part of the spectrum, which come to the Earth from the Sun and heat it. At the same time, it absorbs infrared rays emitted by the Earth and is one of the greenhouse gases, as a result of which it takes part in the process of global warming. A constant increase in the level of this gas in the atmosphere has been observed since the beginning of the industrial era.
Carbon monoxide (IV) - carbon dioxide, an odorless and colorless gas, heavier than air, upon strong cooling it crystallizes in the form of a white snow-like mass - “dry ice”. At atmospheric pressure it does not melt, but evaporates; the sublimation temperature is −78 °C. Carbon dioxide is formed when organic matter rots and burns. Contained in the air and mineral springs, released during the respiration of animals and plants. Soluble in water (1 volume of carbon dioxide in one volume of water at 15 ° C).
According to its chemical properties, carbon dioxide belongs to acidic oxides. When dissolved in water, it forms carbonic acid. Reacts with alkalis to form carbonates and bicarbonates. It undergoes electrophilic substitution reactions (for example, with phenol) and nucleophilic addition (for example, with organomagnesium compounds). Carbon(IV) monoxide does not support combustion. Only some active metals burn in it. Interacts with oxides of active metals. When dissolved in water, it forms carbonic acid. Reacts with alkalis to form carbonates and bicarbonates.
The human body produces approximately 1 kg (2.3 lb) of carbon dioxide per day. This carbon dioxide is transported from the tissues, where it is formed as one of the end products of metabolism, through the venous system and is then excreted in the exhaled air through the lungs. Thus, the content of carbon dioxide in the blood is high in the venous system, and decreases in the capillary network of the lungs, and is low in the arterial blood. The carbon dioxide content of a blood sample is often expressed in terms of partial pressure, that is, the pressure that a given amount of carbon dioxide contained in a blood sample would have if it alone occupied the entire volume of the blood sample. Carbon dioxide ( CO2) is transported in the blood in three different ways (the exact proportion of each of these three transport methods depends on whether the blood is arterial or venous).
- Most of the carbon dioxide (70% to 80%) is converted by the enzyme carbonic anhydrase in red blood cells into bicarbonate ions.
- About 5% - 10% of carbon dioxide is dissolved in blood plasma.
- About 5% - 10% of carbon dioxide is bound to hemoglobin in the form of carbamine compounds (carbohemoglobin).
Hemoglobin, the main oxygen-transporting protein of red blood cells, is capable of transporting both oxygen and carbon dioxide. However, carbon dioxide binds to hemoglobin at a different site than oxygen. It binds to the N-terminal ends of globin chains rather than to heme. However, due to allosteric effects, which lead to a change in the configuration of the hemoglobin molecule upon binding, the binding of carbon dioxide reduces the ability of oxygen to bind to it, at a given partial pressure of oxygen, and vice versa - the binding of oxygen to hemoglobin reduces the ability of carbon dioxide to bind to it, at a given partial pressure of carbon dioxide. In addition, the ability of hemoglobin to preferentially bind with oxygen or carbon dioxide also depends on the pH of the environment. These features are very important for the successful uptake and transport of oxygen from the lungs into the tissues and its successful release into the tissues, as well as for the successful uptake and transport of carbon dioxide from the tissues into the lungs and its release there. Carbon dioxide is one of the most important mediators of autoregulation of blood flow. It is a powerful vasodilator. Accordingly, if the level of carbon dioxide in tissue or blood increases (for example, due to intense metabolism - caused by, say, exercise, inflammation, tissue damage, or due to obstruction of blood flow, tissue ischemia), then the capillaries dilate, which leads to increased blood flow and accordingly, to increase the delivery of oxygen to the tissues and the transport of accumulated carbon dioxide from the tissues. In addition, carbon dioxide in certain concentrations (increased, but not yet reaching toxic values) has a positive inotropic and chronotropic effect on the myocardium and increases its sensitivity to adrenaline, which leads to an increase in the strength and frequency of heart contractions, cardiac output and, as a consequence, , stroke and minute blood volume. This also helps to correct tissue hypoxia and hypercapnia (increased carbon dioxide levels). Bicarbonate ions are very important for regulating blood pH and maintaining normal acid-base balance. Respiration rate affects the carbon dioxide content in the blood. Weak or slow breathing causes respiratory acidosis, while rapid and excessively deep breathing leads to hyperventilation and the development of respiratory alkalosis. In addition, carbon dioxide is also important in regulating respiration. Although our body requires oxygen for metabolism, low oxygen levels in the blood or tissues usually do not stimulate breathing (or rather, the stimulating effect of low oxygen on breathing is too weak and “turns on” late, at very low levels of oxygen in the blood, at which a person often is already losing consciousness). Normally, breathing is stimulated by an increase in the level of carbon dioxide in the blood. The respiratory center is much more sensitive to increased levels of carbon dioxide than to a lack of oxygen. As a consequence, breathing very thin air (with a low partial pressure of oxygen) or a gas mixture containing no oxygen at all (for example, 100% nitrogen or 100% nitrous oxide) can quickly lead to loss of consciousness without causing a feeling of lack of air (because the level of carbon dioxide does not increase in the blood, because nothing prevents its exhalation). This is especially dangerous for pilots of military aircraft flying at high altitudes (if an enemy missile hits the cockpit and the cockpit depressurizes, the pilots can quickly lose consciousness). This feature of the breathing regulation system is also the reason why flight attendants on airplanes instruct passengers in the event of depressurization of the aircraft cabin, first of all, to put on an oxygen mask themselves before trying to help anyone else - by doing this, the helper risks quickly losing consciousness himself, and even without feeling any discomfort or need for oxygen until the last moment. The human respiratory center tries to maintain the partial pressure of carbon dioxide in arterial blood no higher than 40 mmHg. With conscious hyperventilation, the content of carbon dioxide in arterial blood can decrease to 10-20 mmHg, while the oxygen content in the blood will remain virtually unchanged or increase slightly, and the need to take another breath will decrease as a result of a decrease in the stimulating effect of carbon dioxide on the activity of the respiratory center. This is the reason why, after a period of conscious hyperventilation, it is easier to hold your breath for a long time than without previous hyperventilation. This deliberate hyperventilation followed by breath holding can lead to loss of consciousness before the person feels the need to take a breath. In a safe environment, such a loss of consciousness does not threaten anything special (having lost consciousness, a person will lose control over himself, stop holding his breath and take a breath, breathing, and with it the oxygen supply to the brain will be restored, and then consciousness will be restored). However, in other situations, such as before diving, this can be dangerous (loss of consciousness and the need to take a breath will occur at depth, and without conscious control, water will enter the airways, which can lead to drowning). This is why hyperventilation before diving is dangerous and not recommended.
In industrial quantities, carbon dioxide is released from flue gases, or as a by-product of chemical processes, for example, during the decomposition of natural carbonates (limestone, dolomite) or during the production of alcohol (alcoholic fermentation). The mixture of the resulting gases is washed with a solution of potassium carbonate, which absorbs carbon dioxide, turning into bicarbonate. A solution of bicarbonate decomposes when heated or under reduced pressure, releasing carbon dioxide. In modern installations for the production of carbon dioxide, instead of bicarbonate, an aqueous solution of monoethanolamine is more often used, which, under certain conditions, can absorb CO2 contained in the flue gas, and release it when heated; This separates the finished product from other substances. Carbon dioxide is also produced in air separation plants as a by-product of the production of pure oxygen, nitrogen and argon. In the laboratory, small quantities are obtained by reacting carbonates and bicarbonates with acids, such as marble, chalk or soda with hydrochloric acid, using, for example, a Kipp apparatus. Using sulfuric acid to react with chalk or marble results in the formation of slightly soluble calcium sulfate, which interferes with the reaction, and which is removed by a significant excess of acid. To prepare drinks, the reaction of baking soda with citric acid or sour lemon juice can be used. It was in this form that the first carbonated drinks appeared. Pharmacists were engaged in their production and sale.
In the food industry, carbon dioxide is used as a preservative and leavening agent and is indicated on the packaging with code E290. Liquid carbon dioxide is widely used in fire extinguishing systems and fire extinguishers. Automatic carbon dioxide fire extinguishing systems differ in their starting systems, which can be pneumatic, mechanical or electrical. The device for supplying carbon dioxide to the aquarium may include a gas reservoir. The simplest and most common method of producing carbon dioxide is based on the design for making the alcoholic drink mash. During fermentation, the carbon dioxide released may well provide nutrition for aquarium plants. Carbon dioxide is used to carbonate lemonade and sparkling water. Carbon dioxide is also used as a protective medium in wire welding, but at high temperatures it dissociates and releases oxygen. The released oxygen oxidizes the metal. In this regard, it is necessary to introduce deoxidizing agents such as manganese and silicon into the welding wire. Another consequence of the influence of oxygen, also associated with oxidation, is a sharp decrease in surface tension, which leads, among other things, to more intense metal spattering than when welding in an inert environment. Carbon dioxide in cans is used in air guns (in gas-cylinder pneumatics) and as an energy source for engines in aircraft modeling. Storing carbon dioxide in a steel cylinder in a liquefied state is more profitable than in the form of gas. Carbon dioxide has a relatively low critical temperature of +31°C. About 30 kg of liquefied carbon dioxide is poured into a standard 40-liter cylinder, and at room temperature there will be a liquid phase in the cylinder, and the pressure will be approximately 6 MPa (60 kgf/cm²). If the temperature is above +31°C, then carbon dioxide will go into a supercritical state with a pressure above 7.36 MPa. The standard operating pressure for a regular 40-liter cylinder is 15 MPa (150 kgf/cm²), but it must safely withstand pressure 1.5 times higher, that is, 22.5 MPa, so working with such cylinders can be considered quite safe. Solid carbon dioxide - “dry ice” - is used as a refrigerant in laboratory research, in retail trade, during equipment repair (for example: cooling one of the mating parts during a press-fit), etc. Carbon dioxide is used to liquefy carbon dioxide and produce dry ice. installations.
Measuring the partial pressure of carbon dioxide is required in technological processes, in medical applications - analysis of respiratory mixtures during artificial ventilation and in closed life support systems. Concentration Analysis CO2 in the atmosphere is used for environmental and scientific research, to study the greenhouse effect. Carbon dioxide is recorded using gas analyzers based on the principle of infrared spectroscopy and other gas measuring systems. A medical gas analyzer for recording the carbon dioxide content in exhaled air is called a capnograph. For measuring low concentrations CO2(as well as CO) in process gases or in atmospheric air, you can use the gas chromatographic method with a methanator and registration with a flame ionization detector.
Annual fluctuations in the concentration of atmospheric carbon dioxide on the planet are determined mainly by the vegetation of the middle latitudes (40-70°) of the Northern Hemisphere. Vegetation in the tropics is practically independent of the season, the dry desert belt of 20-30° (in both hemispheres) makes a small contribution to the carbon dioxide cycle, and the strips of land most covered with vegetation are located asymmetrically on Earth (in the Southern Hemisphere there is an ocean in the middle latitudes). Therefore, from March to September, due to photosynthesis, the content CO2 in the atmosphere it decreases, and from October to February it increases. Contributions to winter growth come from both the oxidation of wood (heterotrophic respiration of plants, rotting, decomposition of humus, forest fires) and the combustion of fossil fuels (coal, oil, gas), which increases noticeably in the winter season. A large amount of carbon dioxide is dissolved in the ocean. Carbon dioxide makes up a significant part of the atmospheres of some planets in the solar system: Venus, Mars.
Carbon dioxide is non-toxic, but due to the effect of its increased concentrations in the air on air-breathing living organisms, it is classified as asphyxiating gases (English) Russian. Slight increases in concentration up to 2-4% indoors lead to the development of drowsiness and weakness in people. Dangerous concentrations are considered to be levels around 7-10%, at which suffocation develops, manifesting itself in headache, dizziness, hearing loss and loss of consciousness (symptoms similar to those of altitude sickness), depending on the concentration, over a period of several minutes up to one hour. When air with high concentrations of gas is inhaled, death occurs very quickly from suffocation. Although, in fact, even a concentration of 5-7% CO2 is not lethal, already at a concentration of 0.1% (this level of carbon dioxide is observed in the air of megacities) people begin to feel weak and drowsy. This shows that even at high oxygen levels, high CO2 concentrations have a significant impact on well-being. Inhalation of air with an increased concentration of this gas does not lead to long-term health problems, and after removing the victim from the polluted atmosphere, complete restoration of health quickly occurs.
Application of carbonic acid (carbon dioxide)
Currently, carbon dioxide in all its states is widely used in all sectors of industry and the agro-industrial complex.
In gaseous state (carbon dioxide)
In the food industry
1. To create an inert bacteriostatic and fungistatic atmosphere (at concentrations above 20%):
· when processing plant and animal products;
· when packaging food products and medicines to significantly increase their shelf life;
· when dispensing beer, wine and juices as a displacing gas.
2. In the production of soft drinks and mineral waters (saturation).
3. In brewing and production of champagne and sparkling wines (carbonation).
4. Preparation of carbonated water and drinks using siphons and saturators, for personnel in hot shops and in the summer.
5. Use in vending machines for the sale of bottled gas and water and for the manual sale of beer and kvass, carbonated water and drinks.
6. In the production of carbonated milk drinks and carbonated fruit and berry juices (“sparkling products”).
7. In the production of sugar (defecation - saturation).
8. For long-term preservation of fruit and vegetable juices while preserving the smell and taste of a freshly squeezed product by saturating with CO2 and storing under high pressure.
9. To intensify the processes of precipitation and removal of tartaric acid salts from wines and juices (detartation).
10. For the preparation of drinking desalinated water using the filtration method. To saturate salt-free drinking water with calcium and magnesium ions.
In the production, storage and processing of agricultural products
11. To increase the shelf life of food products, vegetables and fruits in a controlled atmosphere (2-5 times).
12. Storing cut flowers for 20 days or more in a carbon dioxide atmosphere.
13. Storing cereals, pasta, grains, dried fruits and other food products in a carbon dioxide atmosphere to protect them from damage by insects and rodents.
14. For treating fruits and berries before storing, which prevents the development of fungal and bacterial rot.
15. For high-pressure saturation of cut or whole vegetables, which enhances flavor notes (“sparkling products”) and improves their shelf life.
16. To improve growth and increase productivity of plants in protected soil.
Today, in vegetable and flower growing farms in Russia, the issue of fertilizing plants in protected soil with carbon dioxide is an urgent issue. CO2 deficiency is a more serious problem than deficiency of mineral nutrients. On average, a plant synthesizes 94% of its dry matter mass from water and carbon dioxide; the plant receives the remaining 6% from mineral fertilizers! Low carbon dioxide content is now a factor limiting yield (primarily in low-volume crops). The air in a 1-hectare greenhouse contains about 20 kg of CO2. At maximum lighting levels in the spring and summer months, CO2 consumption by cucumber plants during photosynthesis can approach 50 kg h/ha (i.e., up to 700 kg/ha CO2 per daylight hours). The resulting deficit is only partially covered by the influx of atmospheric air through the transoms and the leakage of the enclosing structures, as well as by the night respiration of plants. In ground greenhouses, an additional source of carbon dioxide is soil filled with manure, peat, straw or sawdust. The effect of enriching greenhouse air with carbon dioxide depends on the amount and type of these organic substances that undergo microbiological decomposition. For example, when adding sawdust moistened with mineral fertilizers, the level of carbon dioxide at first can reach high values at night, and during the day when the transoms are closed. However, in general, this effect is not great enough and satisfies only part of the plants’ needs. The main disadvantage of biological sources is the short duration of increasing the concentration of carbon dioxide to the desired level, as well as the impossibility of regulating the feeding process. Often in ground greenhouses on sunny days with insufficient air exchange, the CO2 content as a result of intensive absorption by plants can fall below 0.01% and photosynthesis practically stops! Lack of CO2 becomes the main factor limiting the assimilation of carbohydrates and, accordingly, the growth and development of plants. It is possible to completely cover the deficit only through the use of technical sources of carbon dioxide.
17. Production of microalgae for livestock. When water is saturated with carbon dioxide in installations for autonomous algae cultivation, the algae growth rate increases significantly (4-6 times).
18. To improve the quality of silage. When ensiling succulent feed, the artificial introduction of CO2 into the plant mass prevents the penetration of oxygen from the air, which contributes to the formation of a high-quality product with a favorable ratio of organic acids, a high content of carotene and digestible protein.
19. For safe disinfestation of food and non-food products. An atmosphere containing more than 60% carbon dioxide within 1-10 days (depending on temperature) destroys not only adult insects, but their larvae and eggs. This technology is applicable to products with bound water content up to 20%, such as grain, rice, mushrooms, dried fruits, nuts and cocoa, animal feed and much more.
20. For the total destruction of mouse-like rodents by briefly filling burrows, storage facilities, and chambers with gas (a sufficient concentration of 30% carbon dioxide).
21. For anaerobic pasteurization of animal feed, mixed with water vapor at a temperature not exceeding 83 degrees C - as a replacement for granulation and extrusion, which does not require large energy costs.
22. For euthanizing poultry and small animals (pigs, calves, sheep) before slaughter. For anesthesia of fish during transportation.
23. For anesthesia of queen bees and bumblebees in order to accelerate the onset of oviposition.
24. To saturate drinking water for chickens, which significantly reduces the negative impact of elevated summer temperatures on poultry, helps thicken egg shells and strengthen the bones.
25. To saturate working solutions of fungicides and herbicides for better action of the preparations. This method allows you to reduce solution consumption by 20-30%.
In medicine
26. a) mixed with oxygen as a respiratory stimulant (at a concentration of 5%);
b) for dry carbonated baths (at a concentration of 15-30%) in order to lower blood pressure and improve blood flow.
27. Cryotherapy in dermatology, dry and water carbon dioxide baths in balneotherapy, breathing mixtures in surgery.
In the chemical and paper industries
28. For the production of soda, ammonium carbon salts (used as fertilizers in crop production, additives in ruminant animal feed, instead of yeast in baked goods and flour confectionery), white lead, urea, hydroxycarboxylic acids. For the catalytic synthesis of methanol and formaldehyde.
29. For neutralization of alkaline wastewater. Due to the self-buffering effect of the solution, precise pH regulation avoids corrosion of equipment and waste pipes, and there is no formation of toxic by-products.
30. In the production of paper for processing pulp after alkaline bleaching (increases the efficiency of the process by 15%).
31. To increase the yield and improve the physical and mechanical properties and bleachability of cellulose during oxygen-soda cooking of wood.
32. To clean heat exchangers from scale and prevent its formation (a combination of hydrodynamic and chemical methods).
In construction and other industries
33. For rapid chemical hardening of molds for steel and cast iron castings. The supply of carbon dioxide to casting molds accelerates their hardening 20-25 times compared to thermal drying.
34. As a foaming gas in the production of porous plastics.
35. For strengthening refractory bricks.
36. For semi-automatic welding machines for repairing bodies of passenger and passenger cars, repairing cabins of trucks and tractors and for electric welding of thin-sheet steel products.
37. In the manufacture of welded structures with automatic and semi-automatic electric welding in an environment of carbon dioxide as a protective gas. Compared to welding with a stick electrode, the convenience of work increases, productivity increases by 2-4 times, the cost of 1 kg of deposited metal in a CO2 environment is more than two times lower compared to manual arc welding.
38. As a protective medium in mixtures with inert and noble gases during automated welding and metal cutting, thanks to which very high quality seams are obtained.
39. Charging and recharging of fire extinguishers, for fire-fighting equipment. In fire extinguishing systems, for filling fire extinguishers.
40. Charging cans for gas weapons and siphons.
41. As a nebulizer gas in aerosol cans.
42. For filling sports equipment (balls, balls, etc.).
43. As an active medium in medical and industrial lasers.
44. For precise calibration of instruments.
In the mining industry
45. For softening of the coal rock mass during the mining of hard coal in rock-prone formations.
46. For carrying out blasting operations without creating a flame.
47. Increasing the efficiency of oil production by adding carbon dioxide to oil reservoirs.
In liquid state (low temperature carbon dioxide)
In the food industry
1. For quick freezing, to a temperature of -18 degrees C and below, of food products in contact freezers. Along with liquid nitrogen, liquid carbon dioxide is most suitable for direct contact freezing of various types of products. As a contact refrigerant, it is attractive due to its low cost, chemical passivity and thermal stability, does not corrode metal components, is not flammable, and is not dangerous to personnel. Liquid carbon dioxide is supplied to the product moving on the conveyor belt from the nozzles in certain portions, which at atmospheric pressure instantly turns into a mixture of dry snow and cold carbon dioxide, while fans constantly mix the gas mixture inside the apparatus, which, in principle, is capable of cooling the product from +20 degrees. C to -78.5 degrees C in a few minutes. The use of contact quick freezers has a number of fundamental advantages compared to traditional freezing technology:
Freezing time is reduced to 5-30 minutes; enzymatic activity in the product quickly ceases;
· the structure of tissues and cells of the product is well preserved, since ice crystals are formed of much smaller sizes and almost simultaneously in the cells and in the intercellular space of tissues;
· with slow freezing, traces of bacterial activity appear in the product, while with shock freezing they simply do not have time to develop;
· product weight loss as a result of shrinkage is only 0.3-1% (versus 3-6%);
· Easily volatile valuable aromatic substances will be preserved in much larger quantities. Compared to freezing with liquid nitrogen, freezing with carbon dioxide:
· cracking of the product is not observed due to too large a temperature difference between the surface and the core of the frozen product
· during the freezing process, CO2 penetrates into the product and during defrosting it protects it from oxidation and the development of microorganisms. Fruits and vegetables subjected to quick freezing and packaging on site most fully retain their taste and nutritional value, all vitamins and biologically active substances, which makes it possible to widely use them for the production of products for children's and dietary nutrition. It is important that non-standard fruit and vegetable products can be successfully used to prepare expensive frozen mixtures. Quick-freezers using liquid carbon dioxide are compact, simple in design and inexpensive to operate (if there is a nearby source of cheap liquid carbon dioxide). The devices exist in mobile and stationary versions, spiral, tunnel and cabinet types, which are of interest to agricultural producers and product processors. They are especially convenient when production requires freezing of various food products and raw materials at different temperature conditions (-10...-70 degrees C). Quick-frozen foods can be dried under high vacuum conditions - freeze drying. Products dried using this method are of high quality: they retain all nutrients, have increased restorative capacity, have minimal shrinkage and porous structure, and retain their natural color. Freeze-dried products are 10 times lighter than the original ones due to the removal of water from them, they are stored for a very long time in sealed bags (especially when the bags are filled with carbon dioxide) and can be cheaply delivered to the most remote areas.
2. For rapid cooling of fresh food products, packaged and unpackaged, to +2…+6 degrees C. Using installations whose operation is similar to the operation of quick-freezers: when liquid carbon dioxide is injected, tiny dry snow is formed, with which the product is processed for a certain time. Dry snow is an effective means of quickly reducing temperature, which does not lead to drying out of the product, like air cooling, and does not increase its moisture content, as happens when cooling with water ice. Dry snow cooling provides the required temperature reduction in just a few minutes, rather than the hours required with conventional cooling. The natural color of the product is preserved and even improved due to the slight diffusion of CO2 inside. At the same time, the shelf life of products increases significantly, since CO2 suppresses the development of both aerobic and anaerobic bacteria and mold fungi. It is convenient and profitable to refrigerate poultry meat (cut or in carcasses), portioned meat, sausages and semi-finished products. The units are also used where the technology requires rapid cooling of the product during or before molding, pressing, extruding, grinding or slicing. Devices of this type are also very convenient for use in poultry farms for in-line ultra-fast cooling from 42.7 degrees C to 4.4-7.2 degrees C of freshly laid chicken eggs.
3. To remove the skin from berries using the freezing method.
4. For cryopreservation of sperm and embryos of cattle and pigs.
In the refrigeration industry
5. For use as an alternative refrigerant in refrigeration systems. Carbon dioxide can serve as an effective refrigerant because it has a low critical temperature (31.1 degrees C), a relatively high triple point temperature (-56 degrees C), a high triple point pressure (0.5 mPa) and a high critical pressure ( 7.39 mPa). As a refrigerant it has the following advantages:
· very low price compared to other refrigerants;
· non-toxic, non-flammable and non-explosive;
· compatible with all electrical insulating and structural materials;
· does not destroy the ozone layer;
· makes a moderate contribution to the increase in the greenhouse effect compared to modern halogenated refrigerants. A high critical pressure has the positive aspect of a low compression ratio, resulting in significant compressor efficiency, allowing for compact and low-cost refrigeration designs. At the same time, additional cooling of the condenser electric motor is required, and the metal consumption of the refrigeration unit increases due to the increase in the thickness of the pipes and walls. It is promising to use CO2 in low-temperature two-stage installations for industrial and semi-industrial applications, and especially in air conditioning systems for cars and trains.
6. For high-performance frozen grinding of soft, thermoplastic and elastic products and substances. In cryogenic mills, those products and substances that cannot be ground in their usual form, for example gelatin, rubber, any polymers, tires, are ground quickly and with low energy consumption in frozen form. Cold grinding in a dry, inert atmosphere is necessary for all herbs and spices, cocoa beans and coffee beans.
7. For testing technical systems at low temperatures.
In metallurgy
8. For cooling difficult-to-cut alloys when processed on lathes.
9. To form a protective environment for smoke suppression in copper, nickel, zinc and lead smelting or bottling processes.
10. When annealing solid copper wire for cable products.
In the mining industry
11. As a low-blasting explosive in coal mining, which does not lead to the ignition of methane and coal dust during an explosion, and does not produce toxic gases.
12. Prevention of fires and explosions by displacing air from containers and mines containing explosive vapors and gases with carbon dioxide.
Supercritical
In extraction processes
1. Capturing aromatic substances from fruit and berry juices, obtaining plant extracts and medicinal herbs using liquid carbon dioxide. In traditional methods of extraction of plant and animal raw materials, various types of organic solvents are used, which are highly specific and rarely ensure the extraction of the full complex of biologically active compounds from raw materials. Moreover, the problem of separating solvent residues from the extract always arises, and the technological parameters of this process can lead to partial or even complete destruction of some components of the extract, which causes a change not only in the composition, but also in the properties of the isolated extract. Compared to traditional methods, extraction processes (as well as fractionation and impregnation) using supercritical carbon dioxide have a number of advantages:
· energy-saving nature of the process;
· high mass transfer characteristics of the process due to low viscosity and high penetrating ability of the solvent;
· high degree of extraction of relevant components and high quality of the resulting product;
· virtual absence of CO2 in finished products;
· an inert dissolving medium is used at a temperature that does not threaten thermal degradation of materials;
· the process does not produce waste water and waste solvents; after decompression, CO2 can be collected and reused;
· the unique microbiological purity of the resulting products is ensured;
· lack of complex equipment and multi-stage process;
· A cheap, non-toxic and non-flammable solvent is used. The selective and extraction properties of carbon dioxide can vary widely with changes in temperature and pressure, which makes it possible to extract most of the spectrum of currently known biologically active compounds from plant materials at low temperatures.
2. To obtain valuable natural products - CO2 extracts of spices, essential oils and biologically active substances. The extract practically copies the original plant material; as for the concentration of its constituent substances, we can state that there are no analogues among classical extracts. Chromatographic analysis data show that the content of valuable substances exceeds classical extracts tens of times. Production on an industrial scale has been mastered:
· extracts from spices and medicinal herbs;
· fruit aromas;
· extracts and acids from hops;
· antioxidants, carotenoids and lycopenes (including from tomato raw materials);
· natural coloring substances (from red pepper fruits and others);
lanolin from wool;
· natural plant waxes;
· sea buckthorn oils.
3. For the extraction of highly purified essential oils, in particular from citrus fruits. When extracting essential oils with supercritical CO2, highly volatile fractions are also successfully extracted, which give these oils fixing properties, as well as a more complete aroma.
4. To remove caffeine from tea and coffee, nicotine from tobacco.
5. To remove cholesterol from food (meat, dairy products and eggs).
6. For the production of low-fat potato chips and soy products;
7. For the production of high-quality tobacco with specified technological properties.
8. For dry cleaning of clothes.
9. To remove uranium compounds and transuranium elements from radioactively contaminated soils and from the surfaces of metal bodies. At the same time, the volume of water waste is reduced hundreds of times, and there is no need to use aggressive organic solvents.
10. For environmentally friendly printed circuit board etching technology for microelectronics, without generating toxic liquid waste.
In fractionation processes
The separation of a liquid substance from a solution, or the separation of a mixture of liquid substances is called fractionation. These processes are continuous and therefore much more efficient than the separation of substances from solid substrates.
11. For refining and deodorizing oils and fats. To obtain commercial oil, it is necessary to carry out a whole range of measures, such as removing lecithin, mucus, acid, bleaching, deodorization and others. When extracting with supercritical CO2, these processes are carried out during one technological cycle, and the quality of the oil obtained in this case is much better, since the process takes place at relatively low temperatures.
12. To reduce the alcohol content in drinks. The production of non-alcoholic traditional drinks (wine, beer, cider) is in increasing demand for ethical, religious or dietary reasons. Even though these low-alcohol drinks are often of lower quality, their market is significant and growing rapidly, so improving such technology is a very attractive issue.
13. For energy-saving production of high-purity glycerin.
14. For energy-saving production of lecithin from soybean oil (with a phosphatidyl choline content of about 95%).
15. For flow-through purification of industrial wastewater from hydrocarbon pollutants.
In impregnation processes
The process of impregnation - the introduction of new substances, is essentially the reverse process of extraction. The required substance is dissolved in supercritical CO2, then the solution penetrates into the solid substrate, when the pressure is released, the carbon dioxide instantly evaporates, and the substance remains in the substrate.
16. For environmentally friendly dyeing technology for fibers, fabrics and textile accessories. Painting is a special case of impregnation. Dyes are usually dissolved in a toxic organic solvent, so dyed materials must be thoroughly washed, causing the solvent to either evaporate into the atmosphere or end up in wastewater. In supercritical dyeing, water and solvents are not used; the dye is dissolved in supercritical CO2. This method provides an interesting opportunity to dye different types of synthetic materials at the same time, such as plastic teeth and the fabric lining of a zipper.
17. For environmentally friendly technology, paint application. The dry dye dissolves in a stream of supercritical CO2, and along with it flies out of the nozzle of a special gun. Carbon dioxide immediately evaporates, and the paint settles on the surface. This technology is especially promising for painting cars and large equipment.
18. For homogenized impregnation of polymer structures with drugs, thereby ensuring a constant and prolonged release of the drug in the body. This technology is based on the ability of supercritical CO2 to easily penetrate many polymers, saturate them, causing micropores to open and swell.
In technological processes
19. Replacing high-temperature water vapor with supercritical CO2 in extrusion processes, when processing grain-like raw materials, allows the use of relatively low temperatures, the introduction of dairy ingredients and any heat-sensitive additives into the recipe. Supercritical fluid extrusion allows the creation of new products with an ultra-porous internal structure and a smooth, dense surface.
20. For the production of polymer and fat powders. A stream of supercritical CO2 with some polymers or fats dissolved in it is injected into a chamber with lower pressure, where they are “condensed” in the form of a completely homogeneous finely dispersed powder, the finest fibers or films.
21. To prepare for drying greens and fruits by removing the cuticular wax layer with a jet of supercritical CO2.
In chemical reaction processes
22. A promising area of application of supercritical CO2 is its use as an inert medium during chemical reactions of polymerization and synthesis. In a supercritical environment, synthesis can occur a thousand times faster than the synthesis of the same substances in traditional reactors. It is very important for industry that such a significant acceleration of the reaction rate, due to high concentrations of reagents in a supercritical medium with its low viscosity and high diffusivity, makes it possible to correspondingly reduce the contact time of the reagents. In technological terms, this makes it possible to replace static closed reactors with flow reactors that are fundamentally smaller, cheaper and safer.
In thermal processes
23. As a working fluid for modern power plants.
24. As a working fluid of gas heat pumps producing high-temperature heat for hot water supply systems.
In solid state (dry ice and snow)
In the food industry
1. For contact freezing of meat and fish.
2. For contact quick freezing of berries (red and black currants, gooseberries, raspberries, chokeberries and others).
3. Sales of ice cream and soft drinks in places remote from the power grid, cooled with dry ice.
4. When storing, transporting and selling frozen and chilled food products. The production of briquetted and granulated dry ice for buyers and sellers of perishable products is being developed. Dry ice is very convenient for transportation and for selling meat, fish, and ice cream in hot weather - the products remain frozen for a very long time. Since dry ice only evaporates (sublimates), there is no melted liquid, and transport containers always remain clean. Autorefrigerators can be equipped with a small-sized dry-ice cooling system, which is characterized by extreme simplicity of the device and high operational reliability; its cost is many times lower than the cost of any classical refrigeration unit. When transporting over short distances, such a cooling system is the most economical.
5. To pre-cool containers before loading products. Blowing dry snow in cold carbon dioxide is one of the most effective ways to pre-cool any containers.
6. For air transportation as a primary refrigerant in isothermal containers with an autonomous two-stage refrigeration system (granulated dry ice - freon).
During surface cleaning work
8. Cleaning of parts and components, engines from contaminants using treatment plants using dry ice granules in a gas flow. To clean the surfaces of components and parts from operational contaminants. Recently, there has been a great demand for non-abrasive express cleaning of materials, dry and wet surfaces with a jet of finely granulated dry ice (blasting). Without disassembling the units, you can successfully carry out:
· cleaning of welding lines;
· removal of old paint;
· cleaning of foundry molds;
· cleaning of printing machine units;
· cleaning of equipment for the food industry;
· cleaning of molds for the production of polyurethane foam products.
· cleaning of molds for the production of car tires and other rubber products;
· cleaning of molds for the production of plastic products, including cleaning of molds for the production of PET bottles; When dry ice pellets hit a surface, they instantly evaporate, creating a micro-explosion that removes contaminants from the surface. When removing brittle material such as paint, the process creates a pressure wave between the coating and the substrate. This wave is strong enough to remove the coating, lifting it from the inside. When removing sticky or sticky materials such as oil or dirt, the cleaning process is similar to a strong jet of water.
7. For cleaning stamped rubber and plastic products from burrs (tumbling).
During construction work
9. In the process of manufacturing porous building materials with the same size of carbon dioxide bubbles, evenly distributed throughout the entire volume of the material.
10. For freezing soils during construction.
11. Installation of ice plugs in pipes with water (by freezing them from the outside with dry ice), during repair work on pipelines without draining the water.
12. For cleaning artesian wells.
13. When removing asphalt surfaces in hot weather.
In other industries
14. Receiving low temperatures down to minus 100 degrees (when mixing dry ice with ether) for testing product quality, for laboratory work.
15. For cold fitting of parts in mechanical engineering.
16. In the production of ductile grades of alloy and stainless steels, annealed aluminum alloys.
17. When crushing, grinding and preserving calcium carbide.
18. To create artificial rain and obtain additional precipitation.
19. Artificial dispersal of clouds and fog, combating hail.
20. To generate harmless smoke during performances and concerts. Obtaining a smoke effect on pop stages during artist performances using dry ice.
In medicine
21. For the treatment of certain skin diseases (cryotherapy).
Carbon dioxide is a colorless gas with a barely perceptible odor, non-toxic, heavier than air. Carbon dioxide is widely distributed in nature. It dissolves in water, forming carbonic acid H 2 CO 3, giving it a sour taste. The air contains about 0.03% carbon dioxide. The density is 1.524 times greater than the density of air and is equal to 0.001976 g/cm 3 (at zero temperature and pressure 101.3 kPa). Ionization potential 14.3V. Chemical formula – CO 2 .
In welding production the term is used "carbon dioxide" cm. . In the “Rules for the Design and Safe Operation of Pressure Vessels” the term "carbon dioxide", and in - term "carbon dioxide".
There are many ways to produce carbon dioxide, the main ones are discussed in the article.
The density of carbon dioxide depends on pressure, temperature and the state of aggregation in which it is found. At atmospheric pressure and a temperature of -78.5°C, carbon dioxide, bypassing the liquid state, turns into a white snow-like mass "dry ice".
Under a pressure of 528 kPa and at a temperature of -56.6 ° C, carbon dioxide can be in all three states (the so-called triple point).
Carbon dioxide is thermally stable, dissociating into carbon monoxide only at temperatures above 2000°C.
Carbon dioxide is first gas to be described as a discrete substance. In the seventeenth century, a Flemish chemist Jan Baptist van Helmont (Jan Baptist van Helmont) noticed that after burning coal in a closed vessel, the mass of ash was much less than the mass of the burned coal. He explained this by saying that coal was transformed into an invisible mass, which he called “gas.”
The properties of carbon dioxide were studied much later in 1750. Scottish physicist Joseph Black (Joseph Black).
He discovered that limestone (calcium carbonate CaCO 3), when heated or reacted with acids, releases a gas, which he called “bound air”. It turned out that “bound air” is denser than air and does not support combustion.
CaCO 3 + 2HCl = CO 2 + CaCl 2 + H 2 O
By passing “bound air” i.e. carbon dioxide CO 2 through an aqueous solution of lime Ca(OH) 2 calcium carbonate CaCO 3 is deposited to the bottom. Joseph Black used this experiment to prove that carbon dioxide is released through animal respiration.
CaO + H 2 O = Ca(OH) 2
Ca(OH) 2 + CO 2 = CaCO 3 + H 2 O
Liquid carbon dioxide is a colorless, odorless liquid whose density varies greatly with temperature. It exists at room temperature only at pressures above 5.85 MPa. The density of liquid carbon dioxide is 0.771 g/cm 3 (20°C). At temperatures below +11°C it is heavier than water, and above +11°C it is lighter.
The specific gravity of liquid carbon dioxide varies significantly with temperature, therefore, the amount of carbon dioxide is determined and sold by weight. The solubility of water in liquid carbon dioxide in the temperature range 5.8-22.9°C is no more than 0.05%.
Liquid carbon dioxide turns into gas when heat is supplied to it. Under normal conditions (20°C and 101.3 kPa) When 1 kg of liquid carbon dioxide evaporates, 509 liters of carbon dioxide are formed. When gas is withdrawn too quickly, the pressure in the cylinder decreases and the heat supply is insufficient, the carbon dioxide cools, the rate of its evaporation decreases and when it reaches the “triple point” it turns into dry ice, which clogs the hole in the reduction gear, and further gas extraction stops. When heated, dry ice directly turns into carbon dioxide, bypassing the liquid state. To evaporate dry ice, it is necessary to supply significantly more heat than to evaporate liquid carbon dioxide - therefore, if dry ice has formed in the cylinder, it evaporates slowly.
Liquid carbon dioxide was first produced in 1823. Humphry Davy(Humphry Davy) and Michael Faraday(Michael Faraday).
Solid carbon dioxide "dry ice" resembles snow and ice in appearance. The carbon dioxide content obtained from dry ice briquettes is high - 99.93-99.99%. Moisture content is in the range of 0.06-0.13%. Dry ice, being in the open air, evaporates rapidly, so containers are used for its storage and transportation. Carbon dioxide is produced from dry ice in special evaporators. Solid carbon dioxide (dry ice), supplied in accordance with GOST 12162.
Carbon dioxide is most often used:
- to create a protective environment for metals;
- in the production of carbonated drinks;
- refrigeration, freezing and storage of food products;
- for fire extinguishing systems;
- for cleaning surfaces with dry ice.
The density of carbon dioxide is quite high, which allows the arc reaction space to be protected from contact with air gases and prevents nitriding at relatively low carbon dioxide consumption in the jet. Carbon dioxide is, during the welding process, it interacts with the weld metal and has an oxidizing and also carburizing effect on the metal of the weld pool.
Previously obstacles to the use of carbon dioxide as a protective medium were in the seams. The pores were caused by boiling of the solidifying metal of the weld pool from the release of carbon monoxide (CO) due to its insufficient deoxidation.
At high temperatures, carbon dioxide dissociates to form highly active free, monoatomic oxygen:
Oxidation of the weld metal released free from carbon dioxide during welding is neutralized by the content of an additional amount of alloying elements with a high affinity for oxygen, most often silicon and manganese (in excess of the amount required for alloying the weld metal) or fluxes introduced into the welding zone (welding).
Both carbon dioxide and carbon monoxide are practically insoluble in solid and molten metal. The free active oxidizes the elements present in the weld pool depending on their oxygen affinity and concentration according to the equation:
Me + O = MeO
where Me is a metal (manganese, aluminum, etc.).
In addition, carbon dioxide itself reacts with these elements.
As a result of these reactions, when welding in carbon dioxide, significant burnout of aluminum, titanium and zirconium is observed, and less intense burnout of silicon, manganese, chromium, vanadium, etc.
The oxidation of impurities occurs especially vigorously at . This is due to the fact that when welding with a consumable electrode, the interaction of the molten metal with the gas occurs when a drop remains at the end of the electrode and in the weld pool, and when welding with a non-consumable electrode, it occurs only in the pool. As is known, the interaction of gas with metal in an arc gap occurs much more intensely due to the high temperature and larger contact surface of the metal with the gas.
Due to the chemical activity of carbon dioxide in relation to tungsten, welding in this gas is carried out only with a consumable electrode.
Carbon dioxide is non-toxic and non-explosive. At concentrations of more than 5% (92 g/m3), carbon dioxide has a harmful effect on human health, since it is heavier than air and can accumulate in poorly ventilated areas near the floor. This reduces the volume fraction of oxygen in the air, which can cause oxygen deficiency and suffocation. Premises where welding is carried out using carbon dioxide must be equipped with general supply and exhaust ventilation. The maximum permissible concentration of carbon dioxide in the air of the working area is 9.2 g/m 3 (0.5%).
Carbon dioxide is supplied by . To obtain high-quality seams, gaseous and liquefied carbon dioxide of the highest and first grades is used.
Carbon dioxide is transported and stored in steel cylinders or large-capacity tanks in a liquid state, followed by gasification at the plant, with a centralized supply to welding stations through ramps. A standard one with a water capacity of 40 liters is filled with 25 kg of liquid carbon dioxide, which at normal pressure occupies 67.5% of the volume of the cylinder and produces 12.5 m 3 of carbon dioxide upon evaporation. Air accumulates in the upper part of the cylinder along with carbon dioxide gas. Water, which is heavier than liquid carbon dioxide, collects at the bottom of the cylinder.
To reduce the humidity of carbon dioxide, it is recommended to install the cylinder with the valve down and, after settling for 10...15 minutes, carefully open the valve and release moisture from the cylinder. Before welding, it is necessary to release a small amount of gas from a normally installed cylinder to remove any air trapped in the cylinder. Some of the moisture is retained in carbon dioxide in the form of water vapor, worsening the welding of the seam.
When gas is released from the cylinder, due to the throttling effect and heat absorption during the evaporation of liquid carbon dioxide, the gas cools significantly. With intensive gas extraction, the reducer may become clogged with frozen moisture contained in carbon dioxide, as well as dry ice. To avoid this, when extracting carbon dioxide, a gas heater is installed in front of the reducer. The final removal of moisture after the gearbox is carried out with a special desiccant filled with glass wool and calcium chloride, silica gel, copper sulfate or other moisture absorbers
The carbon dioxide cylinder is painted black, with the words “CARBON ACID” written in yellow letters..
A substance with the chemical formula CO2 and a molecular weight of 44.011 g/mol, which can exist in four phase states - gaseous, liquid, solid and supercritical.
The gaseous state of CO2 is commonly called carbon dioxide. At atmospheric pressure it is a colorless, odorless gas, at a temperature of +20? With a density of 1.839 kg/m? (1.52 times heavier than air), dissolves well in water (0.88 volumes in 1 volume of water), partially interacting in it with the formation of carbonic acid. Included in the atmosphere is an average of 0.035% by volume. During sudden cooling due to expansion (expansion), CO2 is able to desublimate - go directly into the solid state, bypassing the liquid phase.
Carbon dioxide gas was previously often stored in stationary gas tanks. Currently, this storage method is not used; carbon dioxide in the required quantity is obtained directly on site - by evaporating liquid carbon dioxide in a gasifier. Then the gas can be easily pumped through any gas pipeline under a pressure of 2-6 atmospheres.
The liquid state of CO2 is technically called “liquid carbon dioxide” or simply “carbon dioxide”. This is a colorless, odorless liquid with an average density of 771 kg/m3, which exists only under a pressure of 3,482...519 kPa at a temperature of 0...-56.5 degrees C (“low-temperature carbon dioxide”), or under a pressure of 3,482...7,383 kPa at a temperature of 0...+31.0 degrees C (“high pressure carbon dioxide”). High-pressure carbon dioxide is most often produced by compressing carbon dioxide to condensation pressure while simultaneously cooling with water. Low-temperature carbon dioxide, which is the main form of carbon dioxide for industrial consumption, is most often produced through a high-pressure cycle by three-stage cooling and throttling in special installations.
For low and medium consumption of carbon dioxide (high pressure), a variety of steel cylinders are used for its storage and transportation (from cylinders for household siphons to containers with a capacity of 55 liters). The most common is a 40 liter cylinder with an operating pressure of 15,000 kPa, containing 24 kg of carbon dioxide. Steel cylinders do not require additional care; carbon dioxide is stored without loss for a long time. High pressure carbon dioxide cylinders are painted black.
For significant consumption, isothermal tanks of various capacities, equipped with service refrigeration units, are used for storing and transporting low-temperature liquid carbon dioxide. There are storage (stationary) vertical and horizontal tanks with a capacity from 3 to 250 tons, transportable tanks with a capacity from 3 to 18 tons. Vertical tanks require the construction of a foundation and are used mainly in conditions of limited space for placement. The use of horizontal tanks makes it possible to reduce the cost of foundations, especially if there is a common frame with a carbon dioxide station. Tanks consist of an internal welded vessel made of low-temperature steel and having polyurethane foam or vacuum thermal insulation; outer casing made of plastic, galvanized or stainless steel; pipelines, fittings and control devices. The internal and external surfaces of the welded vessel are subjected to special treatment, thereby reducing the likelihood of surface corrosion of the metal. In expensive imported models, the outer sealed casing is made of aluminum. The use of tanks ensures filling and draining of liquid carbon dioxide; storage and transportation without product loss; visual control of weight and operating pressure during refueling, during storage and dispensing. All types of tanks are equipped with a multi-level security system. Safety valves allow inspection and repair without stopping and emptying the tank.
With an instantaneous decrease in pressure to atmospheric pressure, which occurs during injection into a special expansion chamber (throttling), liquid carbon dioxide instantly turns into gas and a thin snow-like mass, which is pressed and carbon dioxide is obtained in a solid state, which is commonly called “dry ice”. At atmospheric pressure, it is a white glassy mass with a density of 1,562 kg/m?, with a temperature of -78.5? C, which in the open air sublimates - gradually evaporates, bypassing the liquid state. Dry ice can also be obtained directly from high-pressure installations used to produce low-temperature carbon dioxide from gas mixtures containing CO2 in an amount of at least 75-80%. The volumetric cooling capacity of dry ice is almost 3 times greater than that of water ice and amounts to 573.6 kJ/kg.
Solid carbon dioxide is usually produced in briquettes measuring 200×100×20-70 mm, in granules with a diameter of 3, 6, 10, 12 and 16 mm, rarely in the form of the finest powder (“dry snow”). Briquettes, granules and snow are stored for no more than 1-2 days in stationary underground mine-type storage facilities, divided into small compartments; transported in special insulated containers with a safety valve. Containers from different manufacturers with a capacity of 40 to 300 kg or more are used. Losses due to sublimation are, depending on the ambient temperature, 4-6% or more per day.
At a pressure above 7.39 kPa and a temperature above 31.6 degrees C, carbon dioxide is in the so-called supercritical state, in which its density is like that of a liquid, and its viscosity and surface tension are like those of a gas. This unusual physical substance (fluid) is an excellent non-polar solvent. Supercritical CO2 is capable of completely or selectively extracting any non-polar constituents with a molecular weight of less than 2,000 daltons: terpenes, waxes, pigments, high molecular weight saturated and unsaturated fatty acids, alkaloids, fat-soluble vitamins and phytosterols. Insoluble substances for supercritical CO2 are cellulose, starch, organic and inorganic high molecular weight polymers, sugars, glycosidic substances, proteins, metals and salts of many metals. Possessing similar properties, supercritical carbon dioxide is increasingly used in the processes of extraction, fractionation and impregnation of organic and inorganic substances. It is also a promising working fluid for modern heat engines.
- Specific gravity. The specific gravity of carbon dioxide depends on the pressure, temperature and state of aggregation in which it is located.
- The critical temperature of carbon dioxide is +31 degrees. Specific gravity of carbon dioxide at 0 degrees and a pressure of 760 mm Hg. equal to 1.9769 kg/m3.
- The molecular weight of carbon dioxide is 44.0. The relative weight of carbon dioxide compared to air is 1.529.
- Liquid carbon dioxide at temperatures above 0 degrees. much lighter than water and can only be stored under pressure.
- The specific gravity of solid carbon dioxide depends on the method of its production. Liquid carbon dioxide, when frozen, turns into dry ice, which is a transparent, glassy solid. In this case, solid carbon dioxide has the highest density (at normal pressure in a vessel cooled to minus 79 degrees, the density is 1.56). Industrial solid carbon dioxide is white in color, its hardness is close to chalk,
- its specific gravity varies depending on the production method in the range of 1.3 - 1.6.
- Equation of state. The relationship between volume, temperature and pressure of carbon dioxide is expressed by the equation
- V= R T/p - A, where
- V - volume, m3/kg;
- R - gas constant 848/44 = 19.273;
- T - temperature, K degrees;
- p pressure, kg/m2;
- A is an additional term characterizing the deviation from the equation of state for an ideal gas. It is expressed by the dependence A = (0.0825 + (1.225)10-7 r)/(T/100)10/3.
- Triple point of carbon dioxide. The triple point is characterized by a pressure of 5.28 ata (kg/cm2) and a temperature of minus 56.6 degrees.
- Carbon dioxide can exist in all three states (solid, liquid and gas) only at the triple point. At pressures below 5.28 ata (kg/cm2) (or at temperatures below minus 56.6 degrees), carbon dioxide can only exist in solid and gaseous states.
- In the vapor-liquid region, i.e. above the triple point, the following relations are valid
- i" x + i"" y = i,
- x + y = 1, where,
- x and y - the proportion of the substance in liquid and vapor form;
- i" is the enthalpy of the liquid;
- i"" - enthalpy of steam;
- i is the enthalpy of the mixture.
- From these values it is easy to determine the values of x and y. Accordingly, for the region below the triple point the following equations will be valid:
- i"" y + i"" z = i,
- y + z = 1, where,
- i"" - enthalpy of solid carbon dioxide;
- z is the fraction of the substance in the solid state.
- At the triple point for three phases there are also only two equations
- i" x + i"" y + i""" z = i,
- x + y + z = 1.
- Knowing the values of i," i"," i""" for the triple point and using the given equations, you can determine the enthalpy of the mixture for any point.
- Heat capacity. The heat capacity of carbon dioxide at a temperature of 20 degrees. and 1 ata is
- Ср = 0.202 and Сv = 0.156 kcal/kg*deg. Adiabatic index k =1.30.
- The heat capacity of liquid carbon dioxide in the temperature range from -50 to +20 degrees. characterized by the following values, kcal/kg*deg. :
- Deg.C -50 -40 -30 -20 -10 0 10 20
- Wed, 0.47 0.49 0.515 0.514 0.517 0.6 0.64 0.68
- Melting point. Melting of solid carbon dioxide occurs at temperatures and pressures corresponding to the triple point (t = -56.6 degrees and p = 5.28 ata) or above it.
- Below the triple point, solid carbon dioxide sublimates. The sublimation temperature is a function of pressure: at normal pressure it is -78.5 degrees, in a vacuum it can be -100 degrees. and below.
- Enthalpy. The enthalpy of carbon dioxide vapor over a wide range of temperatures and pressures is determined using the Planck and Kupriyanov equation.
- i = 169.34 + (0.1955 + 0.000115t)t - 8.3724 p(1 + 0.007424p)/0.01T(10/3), where
- I - kcal/kg, p - kg/cm2, T - degrees K, t - degrees C.
- The enthalpy of liquid carbon dioxide at any point can be easily determined by subtracting the latent heat of vaporization from the enthalpy of saturated vapor. Similarly, by subtracting the latent heat of sublimation, the enthalpy of solid carbon dioxide can be determined.
- Thermal conductivity. Thermal conductivity of carbon dioxide at 0 deg. is 0.012 kcal/m*hour*degree C, and at a temperature of -78 degrees. it drops to 0.008 kcal/m*hour*deg.S.
- Data on the thermal conductivity of carbon dioxide in 10 4 tbsp. kcal/m*hour*degree C at positive temperatures are given in the table.
- Pressure, kg/cm2 10 degrees. 20 deg. 30 deg. 40 degrees
- Carbon dioxide gas
- 1 130 136 142 148
- 20 - 147 152 157
- 40 - 173 174 175
- 60 - - 228 213
- 80 - - - 325
- Liquid carbon dioxide
- 50 848 - - -
- 60 870 753 - -
- 70 888 776 - -
- 80 906 795 670
The thermal conductivity of solid carbon dioxide can be calculated using the formula:
236.5/T1.216 st., kcal/m*hour*deg.S.
- Thermal expansion coefficient. The volumetric expansion coefficient a of solid carbon dioxide is calculated depending on the change in specific gravity and temperature. The linear expansion coefficient is determined by the expression b = a/3. In the temperature range from -56 to -80 degrees. coefficients have the following values: a *10*5st. = 185.5-117.0, b* 10* 5 st. = 61.8-39.0.
- Viscosity. Viscosity of carbon dioxide 10 * 6st. depending on pressure and temperature (kg*sec/m2)
- Pressure, at -15 degrees. 0 deg. 20 deg. 40 degrees
- 5 1,38 1,42 1,49 1,60
- 30 12,04 1,63 1,61 1,72
- 75 13,13 12,01 8,32 2,30
- Dielectric constant. The dielectric constant of liquid carbon dioxide at 50 - 125 ati is in the range of 1.6016 - 1.6425.
- Dielectric constant of carbon dioxide at 15 degrees. and pressure 9.4 - 39 ati 1.009 - 1.060.
- Moisture content of carbon dioxide. The content of water vapor in wet carbon dioxide is determined using the equation,
- X = 18/44 * p’/p - p’ = 0.41 p’/p - p’ kg/kg, where
- p’ - partial pressure of water vapor at 100% saturation;
- p is the total pressure of the steam-gas mixture.
- Solubility of carbon dioxide in water. The solubility of gases is measured by volumes of gas reduced to normal conditions (0 degrees, C and 760 mm Hg) per volume of solvent.
- The solubility of carbon dioxide in water at moderate temperatures and pressures up to 4 - 5 atm obeys Henry's law, which is expressed by the equation
- P = N X, where
- P is the partial pressure of gas above the liquid;
- X is the amount of gas in moles;
- H - Henry's coefficient.
- Liquid carbon dioxide as a solvent. Solubility of lubricating oil in liquid carbon dioxide at a temperature of -20 degrees. up to +25 degrees. is 0.388 g in 100 CO2,
- and increases to 0.718 g per 100 g of CO2 at a temperature of +25 degrees. WITH.
- The solubility of water in liquid carbon dioxide in the temperature range from -5.8 to +22.9 degrees. is no more than 0.05% by weight.
Safety precautions
In terms of the degree of impact on the human body, carbon dioxide gas belongs to the 4th hazard class according to GOST 12.1.007-76 “Harmful substances. Classification and general safety requirements." The maximum permissible concentration in the air of the working area has not been established; when assessing this concentration, one should focus on the standards for coal and ozokerite mines, set within 0.5%.
When using dry ice, when using vessels with liquid low-temperature carbon dioxide, safety measures must be ensured to prevent frostbite on the hands and other parts of the worker’s body.
It is equal to +4), called carbon dioxide (other names: carbon dioxide, carbonic anhydride, carbon dioxide). This substance is usually written with the molecular formula CO2. Its molar mass is 44.01 g/mol. In appearance, under normal conditions, carbonic anhydride is a colorless gas. At low concentrations it is odorless; at higher concentrations it acquires a pungent, sour odor.
For this chemical substance there are three possible states of aggregation, which are characterized by different density values:
- solid (dry ice); at a pressure of 1 atm. and temperature -78.5 °C - 1562 kg/m³;
- liquid (carbon dioxide); at a pressure of 56 atm. and temperature +20 °C - 770 kg/m³;
- gaseous; at a pressure of 1 atm. and temperature 0 °C - 1.977 kg/m³.
The melting point of carbon dioxide is -78 °C, the boiling point is -57 °C. The substance dissolves in water: at 25 °C and a pressure of 100 kPa, its solubility is 1.45 g/l.
Carbon dioxide is a natural chemical compound in the molecule of which oxygen atoms are linked to a carbon atom by a covalent bond. The carbon dioxide molecule is linear and centrosymmetric. Both bonds between carbon and two oxygen atoms are equivalent (essentially double). The molecule is symmetrical about its center, so it has no electric dipole moment.
Carbon dioxide was one of the first gaseous chemical compounds that were no longer identified with air. In the seventeenth century, the Flemish chemist Jan Baptista van Helmont noticed that when he burned coal in a closed vessel, the mass of the resulting ash was much less than that of ordinary carbon dioxide. The properties of carbon dioxide were studied more carefully in 1750 by the Scottish physician Joseph Black.
Carbon dioxide at standard pressure and temperature is found in the Earth's atmosphere in an amount of approximately 0.04% by volume. As part of the carbon cycle, known as photosynthesis, carbon dioxide is absorbed by plants, algae, and cyanobacteria. As a result, water and carbohydrates are formed, but this process occurs only under the influence of light. Carbon dioxide is also produced by the combustion of coal or hydrocarbons, by the fermentation of liquids, and by the exhalation of air by humans and animals. In addition, it is emitted from volcanoes, hot springs, and geysers.
Carbon dioxide plays an important role (absorbs and emits radiation in the thermal infrared range). This chemical compound is also one of the main sources of decrease in ocean pH: when dissolved in water, it forms weak carbonic acid: CO2 + H2O ↔ H2CO3, which is unable to completely dissociate into ions.
Carbon dioxide does not support combustion or respiration. The lit splinter in its atmosphere goes out. Animals and humans suffocate at high concentrations of CO2. At 3% concentration in the air, breathing quickens, at 10%, loss of consciousness and rapid death occur, and at 20%, it causes instant paralysis.
Carbon dioxide is a carbonic anhydride and therefore has the properties of an acidic oxide. In laboratory conditions, it is obtained by reacting chalk with hydrochloric acid in CaCO3 + 2HCl → CaCl2 + CO2 + H2O. In industry, it is produced by the thermal decomposition of limestone or chalk (less commonly magnesite or dolomite): CaCO3 → CaO + CO2. The production of carbon dioxide is a by-product of the low-temperature separation of air into nitrogen and oxygen. Nowadays, special generators are produced to produce carbon dioxide from the air. Such generators are used to supply CO2 to greenhouses in order to create a favorable environment for plants.
Carbon dioxide is widely used in chemical industries. It is used to produce soda, to synthesize organic acids, and to make soft drinks. used as a refrigerant, for example, in winemaking. A carbon dioxide atmosphere is created to prevent rotting of food products, including grapes after they are harvested and before wine production begins.
The production of carbon dioxide or liquefied carbon dioxide is carried out to fill it which is used to extinguish fires. However, they cannot extinguish a person, since a significant part of the stream of liquid CO2 evaporates, while the temperature drops sharply (which can cause frostbite) and the CO2 turns into dry ice. Carbon dioxide is usually used to extinguish electrical wiring. The mechanism is to stop the flow of air oxygen to the source of fire.