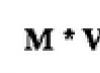Solutions, depending on the type of binder and filler, have different properties and, in this regard, can be used both to connect masonry elements and to obtain a treated surface with certain properties.
Mortars for masonry and installation of wall panels and large blocks. The type and composition of solutions depend on the design stresses and operating conditions. The composition of solutions is usually prescribed using ready-made tables, and they are adjusted based on test results in a construction laboratory.
Laying of above-ground structures operating under low stresses should be done from solutions containing cheap local binders: lime, lime-slag, lime-pozzolanic binder. When laying foundations in aggressive conditions, sulfate-resistant Portland cement is used. For the installation of block and large-panel walls - Portland cement, Portland slag cement, as well as Portland cements with organic additives. The laying of underground structures is usually carried out on cement-based sand solutions without additives of clay or lime. The choice of mobility of mortar mixtures depends on the type of masonry elements and their porosity.
When laying mortars in winter, the hardening rate slows down greatly, so use a mortar that has a grade one or two levels higher than in summer.
Finishing solutions divided into plastering and decorative. The use of these solutions in construction conditions (when plastering wet method) is allowed as an exception. Lime mortars adhere well to the base and change relatively little in volume with fluctuations in temperature and humidity environment. These solutions are recommended for plastering interior walls, partitions, ceilings in rooms with relative humidity air not exceeding 60%, as well as external walls that are not subject to systematic humidification. Lime mortars harden slowly and take a long time to dry.
Cement-lime and cement mortars are used to produce durable, quick-hardening and waterproof plasters. They are used for plastering plinths, cornices, parapets, external walls and other structures that are systematically moistened during operation.
Lime-gypsum mortars are used for plastering internal wooden and stone walls, as well as external walls in areas with a stable dry climate. Such solutions harden very quickly and have great strength with the base, especially with wood.
Decorative solutions and compositions are designed to impart certain architectural and artistic qualities to the facades and interiors of buildings. Depending on the type of finishing, lime-sand, cement-sand, etc., as well as decorative polymer-cement compositions are used. In addition to compressive strength and adhesion to the base, these solutions must retain their original color, texture and other qualities throughout the entire period of operation, regardless of exposure external environment. Therefore, such solutions are subject to increased requirements for frost, light and water resistance.
Waterproofing solutions are used for waterproofing layers, screeds, and plasters. They are made from various types of Portland cement, as well as sulfate-resistant and expanding ones.
Soundproofing (acoustic) solutions are intended for plastering to reduce noise in rooms. They are made using ordinary cement, lime, and gypsum binders. The filler is porous sand made from perlite, expanded clay, pumice, etc., which provides such solutions with open, unclosed porosity and low average density (600-1200 kg/m3).
Unlike commercial mixtures, which are delivered to the construction site in finished form, dry mixtures to give them a marketable state require mixing with water in accordance with the conditions of use and purpose. Typically, recommendations for their use are attached by the manufacturer along with a certificate for the products sold.
The widespread use of dry building mixtures in construction practice is due to:
Stability of the composition, which is ensured in the factory by strict implementation of technological regulations using automatic control means;
Opportunity long-term storage and transportation, including at subzero temperatures;
Wide range of properties of the mortar mixtures used (non-segregation, water-holding capacity) and hardened mortar (better adhesion to the base, adjustable coating strength) due to optimization constituent components, the use of various additives, etc., which cannot always be done under construction conditions;
No losses mortar mixture, which are often observed when using commercial mixtures delivered to construction sites in excess quantities;
Increased labor productivity due to less labor intensity and improved quality of work performed.
Dry mortars have a wider range of applications than conventional mortars. They are used as masonry (for installation of prefabricated elements and laying bricks and blocks, etc.), and plaster solutions(for leveling surfaces, giving them special properties, for example, hydro- and thermal insulation, decorative finishing etc.), as well as for performing various repairs and preparatory work(primer, grout, surface putty, etc.).
The main components of dry mixtures are the following:
Binders - ordinary Portland cement, white and colored, lime - fluff, building gypsum.
Fillers - quartz sand or polymineral sand (without organic or other impurities), of a certain grain composition, finely ground limestone, marble, chalk, tripoli, diatomaceous earth, industrial waste: fly ash, basic slag, etc. For coarse-grained materials, the maximum fineness should be no more than 2, 5 mm, and for fine-grained ones - 0.315 mm.
As additives that perform a stabilizing role (increasing water-holding capacity, cohesiveness, reducing sedimentation phenomena), carboxymethylcellulose (CMC), methylhydroxyethylcellulose, methylcellulose, etc. are used, which are usually dosed in the form of dispersed powders or granules in an amount of 0.1 - 1% of mass of dry mixture.
Dispersible polymer powders (DPP) are produced by spraying and drying latex emulsions. In its own way chemical nature they can be sterol butadiene, vinyl acetate ethylene, vinyl acetate acrylic, etc. Such additives improve mobility and water-holding capacity, adhesion to the surfaces being treated. Their dosage is usually up to 3% by weight of all components.
Depending on the purpose of dry mixtures, various additives (plasticizers, accelerators, pore formers) can be added to their composition, which are currently widely used in the production of concrete and mortars.
Related information.
Solutions in construction – required material for the construction of any buildings: both large and light. These mixtures are divided into several types: mortars are prepared using cement, lime or gypsum base, these substances can also be combined.
There is a gradation according quality indicators, according to the proportions of the binder component and filler, as well as other characteristics.
Types of mortars and their composition
Construction mortar (GOST 5802-78) is a mixture of binder and aggregate (sand) with water. This mixture has the main property of hardening after installation. The mortar is used to bind individual bricks, blocks, stones, etc. together.


The strength of such a bond depends on the quality of the solution used. Application mortars in construction depends on the building material used: for each material it is necessary to use a certain type of mortar.
When constructing showers and toilets, various solutions are used. Depending on the binder included in the mortar, they can be divided into several groups. The main types of mortars in construction are cement, gypsum, lime and combined.


Cement mortars are prepared on the basis of cement or Portland cement. The main component of gypsum solutions is gypsum. Lime mortars contain air or hydraulic lime.


Combined mortars can be prepared on the basis of gypsum and lime, cement and clay, cement and lime, etc.
Lime has more pronounced astringent properties, so all other components are equated to its volume.
For the use of mortars in construction and prepare quality material, guided only by the quantitative ratio of binders and filler, is not always possible, since in addition to such a ratio, it is also necessary to take into account the basic properties of the components, i.e. fat content, brand, amount of impurities, etc.
Simple and complex mortars for construction and their proportions
The durability of the design of the summer shower and toilet and their finishing largely depends on the quality of the prepared solution. There are simple and complex mortars for construction: a simple one consists of one binder component and a filler (lime, clay, cement), and a complex one consists of two binder components and a filler (cement-lime).


For simple solutions, designations are used where the mass part of the binder component is indicated in the first place, and the mass part of the filler in the second place (1: 5, etc.).


In complex solutions, the mass parts are indicated in the following sequence: binder, lime paste, filler. The optimal proportions of complex mortars for construction are 1:1:6. The introduction of several binder components affects the structure and properties of the solution. The addition of clay gives greater plasticity to the cement mortar, that is, it acts as a plasticizer.
In complex solutions, the volume of the main binder component is conventionally taken as one. The remaining substances are designated by numbers that indicate how many parts by volume are needed per part of the main binder component. The main astringent component has more pronounced astringent properties compared to other substances included in this solution. Therefore, the name of the solutions is given according to the name of the main binder. So, for example, in the composition of a lime-clay mortar there are two binders- lime and clay.
Fat and lean mortars
There are fatty, lean and normal mortars for construction: each of them has properties that make them suitable or unsuitable for construction work. Fatty solutions are more plastic, but are prone to cracking.


Lean solutions are too hard and therefore do not have sufficient strength. When constructing a summer shower and toilet, it is recommended to use normal mortars, since they have sufficient plasticity and do not crack when drying, and their shrinkage is minimal. To determine the fat content of the solution, just look at the oar with which it is mixed. If the solution only stained the oar, then the solution is thin. Slightly adhering grout is normal, but heavily adhering grout indicates that it is greasy.


Solutions can be divided into heavy ones, whose dry density is more than 1500 kg/m3, and light ones, whose density does not exceed 1500 kg/m3.


According to their intended purpose, mortars are divided into masonry mortars (intended directly for laying brick, stone and stove blocks), finishing mortars (for finishing stoves) and special ones.
Brands of mortars by strength and mobility
Like brick, cement and others used in construction, mortars differ in brand. It is determined depending on the ability of the solution to withstand compressive load. There are the following brands of mortars for masonry: 0, 2, 10, 25, 50, 75, 100, 150, 200. For the construction of a summer shower and toilet, only mortars of grades 150 and 200 are suitable. The strength indicator of the mortar brand is established empirically when testing a cube of it measuring 70 X 70 mm on the 25th day at a temperature of about 20°C. To do this, samples must be taken at different stages of the batch (at the beginning, middle and end).


To uniformly fill vertical and horizontal joints with masonry mortar, it is necessary that it be sufficiently mobile and able to retain moisture. It is clear that these properties depend on the characteristics and ratio of the components. For various works apply various brands mortars by mobility: it can be measured by the depth of immersion in the solution of a standard cone, which has certain parameters. How deeper dive cone, the more mobile the solution is considered. Masonry mortar has a mobility of 9-13 cm for ordinary clay bricks, 7-8 cm for hollow brick, 13-15 cm - for rubble masonry and 5-7 cm - for plastering.
Composition of lime mortar for construction
This solution is prepared from lime paste (1 part), obtained from lime and water, and river sand (2-4 parts). Pour sand into the lime dough while stirring constantly. Mix everything well until a mass of homogeneous consistency is obtained. If the solution sticks to the spatula, it means that it is too greasy.


The degree of fat content can be reduced by introducing an additional amount of sand. If the resulting solution cannot be kept on the spatula when scooping, then add lime. Mortar used for and and internal plastering works, since this is a low grade solution. It does not create problems in operation, since it is characterized by ease of installation and good adhesion.
Cement mortar: composition, properties and preparation
Due to their composition and properties, cement mortars are the most durable; they are capable of hardening both in air and under high humidity and even in water. The beginning of setting of cement mortars begins after approximately 30-40 minutes, and final hardening occurs after 10-12 hours. Due to the high strength properties of cement mortars and their moisture resistance, these materials are used for the construction of capital walls, foundation laying, and the construction of elements of street buildings, most often located in conditions of high humidity or in areas of strong changes in humidity.


When laying a foundation on wet soil and erecting the walls of a summer shower, it is recommended to use mixed cement mortars. They most often consist of two binding elements and filler. An example of such a solution would be a mixture of cement, lime paste and sand. When hardened, such a solution has high strength and moisture resistance. To prepare it you will need 1 part cement, 2 parts lime paste and 6 to 12 parts sand.
To prepare standard cement mortar you need to take cement (1 part), river sand(2-5 parts) and water. The ingredients must be combined and then mixed thoroughly. The solution obtained in this way should be used for its intended purpose within an hour. If it is necessary to obtain a particularly plastic mass, it is recommended to reduce the amount of sand to 2-3 parts.


Cement mortar is used for laying walls in winter conditions using the freezing method, erecting walls whose thickness does not exceed 25 cm, and foundations. In addition, cement mortar is recommended for the construction of walls with lightweight brickwork and walls in rooms with high humidity levels.
To obtain cement mortar, cement and sand must be mixed dry and then mixed with water.
Cement-lime and clay mortars: composition, application and how to prepare


The composition of the cement-lime mortar includes cement (1 part), river sand (6-8 parts) and lime dough (2 parts). To prepare it, you first need to combine and mix sand and cement, then add lime paste to the resulting mixture and mix everything thoroughly again until a viscous mass of uniform consistency is obtained. The use of complex cement-lime mortar is recommended for use when construction work Under normal conditions, it is mainly suitable for plastering the yard toilet.


The composition of the lime-clay mortar includes clay dough (1 part) and lime dough (0.4 parts), as well as river sand (4-5 parts). The lime dough must be mixed with the clay dough, and then dry sand must be added to the resulting mixture with constant stirring. After this, you should mix everything and use the solution for its intended purpose.


Compared to cement-lime mortar, cement-clay mortar is considered more durable and quick-setting. In addition, it is easy to transport, as it does not delaminate when shaken.
Cement-clay mortar can be used when working in winter conditions, since the clay retains moisture, which, when defrosted, increases the strength of the mortar. The clay should have a finely ground structure. It should be added in equal proportions with cement.


How to prepare clay mortar for the construction of light structures? To prepare a lime-gypsum-clay solution you will need gypsum (1 part), clay-lime composition (3-4 parts) and water. A large, deep bowl should be filled with water, then pour gypsum into it and mix quickly, then add the clay-gypsum mixture. After this, everything should be thoroughly mixed until a homogeneous mass without lumps is obtained.


Lime-gypsum mortar has higher strength characteristics than limestone.
Depending on the type of work you will need different quantities solution.
Liquid dosage forms(Formae medicamentorum fluidae) They are free disperse systems in which medicinal substances are distributed in a liquid dispersion medium. Medicinal substances in these forms can be in three states of aggregation: liquid, solid and gaseous. Depending on the size of the particles of the dispersed phase and the nature of its connection with the dispersion medium, liquid dosage forms can be true solutions of low-molecular and high-molecular substances, colloidal solutions, suspensions, emulsions and combined systems - usually mixtures of the above systems, most often - extraction dosage forms.
According to medical purposes, liquid dosage forms are divided into dosage forms for internal, external and parenteral (dosage forms for injections) use.
Solutions
Solution (Solutio, Genitive – Solutionis) – a liquid dosage form prepared by dissolving a solid drug substance or liquid in a solvent. Solutions are used for external and internal use, as well as for injection.
The solvent used is:
- distilled water ( Aqua destillata);
- ethanol ( Spiritus aethylicus 70%, 90%, 95%);
- glycerin ( Glycerinurn);
- liquid oils ( Oleum Vaselini, Oleum Olivarum, Oleum Persicorum and etc.).
Accordingly, aqueous, alcoholic, glycerin and oil solutions are isolated. True solutions are always transparent; they should not contain suspended particles or sediment. Solutions are used for external and internal use, as well as for injection.
Solutions for external use are solutions that are used as eye and ear drops, nasal drops, as well as for lotions, rinsing, and douching.
A solution in drops is prescribed in a volume of 5-10 ml, solutions for other purposes - in an amount of 50-100 ml or more. Presented in the recipe in shortened or expanded form.
When using the abbreviated form of the copybook after the letters Rp.: indicate the name dosage form, then the name of the medicinal substance, the concentration of the solution and its amount in milliliters. The concentration of the solution is indicated by:
- as a percentage (most often);
- in relationships (1: 1000; 1: 5000);
- in mass-volume ratios (0.1 – 200 ml).
An abbreviated form of prescription of solutions is used in cases where
when the choice of solvent is determined by factory technology or provided to the pharmacy worker. If the solution is aqueous, then the type of solvent is not indicated in the abbreviated recipe. If the solution is oily or alcoholic, then the name of the medicinal substance is followed by the designations - oleosae(oil) or spirituosae(alcohol).
Solutions are a homogeneous mass or mixture consisting of two or more substances, in which one substance acts as a solvent and the other as soluble particles.
There are two theories for interpreting the origin of solutions: chemical, the founder of which is D.I. Mendeleev, and physical, proposed by the German and Swiss physicists Ostwald and Arrhenius. According to Mendeleev's interpretation, the components of the solvent and the dissolved substances become participants in a chemical reaction with the formation of unstable compounds of these same components or particles.
The physical theory denies the chemical interaction between the molecules of the solvent and the dissolved substances, explaining the process of formation of solutions as a uniform distribution of particles (molecules, ions) of the solvent between the particles of the dissolved substance due to physical phenomenon, called diffusion.
Classification of solutions according to various criteria
Today there is no unified system for classifying solutions, however, conditionally, types of solutions can be grouped according to the most significant criteria, namely:
I) Based on their state of aggregation, they are divided into: solid, gaseous and liquid solutions.
II) According to the size of the particles of the dissolved substance: colloidal and true.
III) According to the degree of concentration of the particles of the dissolved substance in the solution: saturated, unsaturated, concentrated, dilute.
IV) According to their ability to conduct electric current: electrolytes and non-electrolytes.
V) By purpose and area of application: chemical, medical, construction, special solutions, etc.
Types of solutions by state of aggregation
The classification of solutions according to the aggregative state of the solvent is given in the broad sense of the meaning of this term. It is customary to consider liquid substances as solutions (and both a liquid and a solid element can act as a soluble substance), however, if we take into account the fact that a solution is a homogeneous system of two or more substances, then it is quite logical to recognize also solid solutions and gaseous. Solid solutions are considered to be mixtures, for example, of several metals, more commonly known as alloys. Gaseous types of solutions are mixtures of several gases, an example is the air around us, which is presented in the form of a compound of oxygen, nitrogen and carbon dioxide.
Solutions by size of dissolved particles
Types of solutions based on the size of dissolved particles include true (ordinary) solutions and B. The solute disintegrates into small molecules or atoms, similar in size to solvent molecules. At the same time, true types of solutions retain the original properties of the solvent, only slightly transforming it under the influence physical and chemical properties element added to it. For example: when table salt or sugar is dissolved in water, the water remains in the same state of aggregation and the same consistency, almost the same color, only its taste changes.

Colloidal solutions differ from ordinary ones in that the added component does not completely disintegrate, preserving complex molecules and compounds, the sizes of which significantly exceed the solvent particles, exceeding the value of 1 nanometer.
Types of solution concentrations
You can add different amounts of the dissolved element to the same amount of solvent, and the output will be solutions with different concentrations. We list the main ones:
- Saturated solutions are characterized by the degree to which the soluble component, under the influence of a constant temperature and pressure, no longer disintegrates into atoms and molecules and the solution reaches phase equilibrium. Saturated solutions can also be divided into concentrated ones, in which the dissolved component is comparable to the solvent, and dilute ones, where the dissolved substance is several times less than the solvent.
- Unsaturated solutions are those in which the solute can still disintegrate into small particles.
- Supersaturated solutions are obtained when the parameters of the influencing factors (temperature, pressure) change, as a result of which the process of “crushing” the dissolved substance continues, it becomes larger than it was under normal (usual) conditions.
Electrolytes and non-electrolytes
Some substances in solutions break down into ions that can conduct electric current. Such homogeneous systems are called electrolytes. This group includes acids and most salts. And solutions that do not conduct electric current are usually called non-electrolytes (almost all organic compounds).

Groups of solutions by purpose
Solutions are indispensable in all sectors of the national economy, the specificity of which has created such types of special solutions as medical, construction, chemical and others.
Medical solutions are a set of preparations in the form of ointments, suspensions, mixtures, solutions for infusions and injections and other dosage forms used in medical purposes for the treatment and prevention of various diseases.

Types of chemical solutions include a huge variety of homogeneous compounds used in chemical reactions: acids, salts. These solutions can be of organic or inorganic origin, aqueous ( sea water) or anhydrous (based on benzene, acetone, etc.), liquid (vodka) or solid (brass). They have found their application in the most various industries national economy: chemical, food, textile industries.
Types of mortars have a viscous and thick consistency, which is why the name of the mixture is more suitable for them.

Due to their ability to quickly harden, they are successfully used as masonry for walls, ceilings, load-bearing structures, as well as for finishing works. Represent aqueous solutions, most often three-component (solvent, cement of various markings, filler), where sand, clay, crushed stone, lime, gypsum and other building materials are used as filler.
Send your good work in the knowledge base is simple. Use the form below
Students, graduate students, young scientists who use the knowledge base in their studies and work will be very grateful to you.
Ministry of Education of the Russian Federation
Penza State University
Medical Institute
Department of Therapy
Essay
" Solutions,used in ITT"
Penza2008
Plan
1. Crystalloid solutions
2. Replacement solutions
3. Basic solutions
4. Corrective solutions
Literature
1. Crystalloid solutions
This group includes infusion solutions of electrolytes and sugars. With the help of these solutions, the basic (physiological) need for water and electrolytes is provided and the violations of water, electrolyte and acid-base balance are corrected. Unlike colloidal solutions, most crystalloid solutions quickly leave the vascular bed and move into the interstitium or cells, depending on their composition.
Conventionally, infusion solutions of electrolytes and sugars (glucose or fructose) can be divided into three groups:
1) replacement solutions (used to replace the loss of blood, water and electrolytes);
2) basic solutions (providing physiological needs for water and electrolytes);
3) correcting solutions (used to correct the imbalance of ions, water and CBS).
2. Replacement solutions
To replenish the isotonic volume deficit, polyelectrolyte solutions are used, the osmolarity and composition of which are close to those of plasma and extracellular fluid. The optimal solutions for this purpose are isotonic and isoionic solutions with a balanced composition. Unfortunately, only a few solutions have such properties. However, experience shows that the use of even unbalanced solutions (Ringer's solution, isotonic sodium chloride solution) in acute situations gives positive results. The main criteria for these solutions should be isotonicity or moderate hypertonicity, and a sufficient content of ingredients that make up the extracellular environment.
Isotonic (0.85-0.9%) sodium chloride solution (saline) was the first solution used to treat blood loss and dehydration.
1 liter of solution contains: Na + - 154 mmol, C1 - 154 mmol. Total osmolarity is 308 mOsm/L, which is slightly higher than plasma osmolarity. pH 5.5 - 7.0. The concentration of chlorine in the solution is also higher than the concentration of this ion in the plasma. Therefore, it cannot be considered absolutely physiological.
It is used mainly as a sodium and chlorine donor for loss of extracellular fluid. It is also indicated for hypochloremia with metabolic alkalosis, oliguria due to dehydration and hyponatremia. The solution combines well with all blood substitutes and blood. It should not be mixed with erythromycin, oxacillin and penicillin. It cannot be used as a universal solution, since it contains little free water and no potassium; acidic solution, increases hypokalemia. Contraindicated in hypernatremia and hyperchloremia.
The total dose is up to 2 liters per day. It is administered intravenously, the infusion rate is 4-8 ml/kg body weight per hour.
Ringer's solution- isotonic electrolyte solution, 1 liter of which contains: Na + - 140 mmol, K + - 4 mmol, Ca 2+ - 6 mmol, Cl - 150 mmol. Osmolarity 300 mOsm/l. This solution has been used as a blood substitute since the end of the last century. Ringer's solution and its modifications are widely used today. This is a physiological replacement solution with mild acidic properties.
Used to replace the loss of extracellular fluid, including blood, and as a carrier solution for electrolyte concentrates. Contraindicated in hyperchloremia and hypernatremia. It should not be mixed with phosphate-containing electrolyte concentrates.
Dose - up to 3000 ml/day in the form of a continuous intravenous drip infusion at an administration rate of 120-180 drops/min at 70 kg body weight.
Saline infusin CIPC is an isotonic electrolyte solution containing various salts. Created during the Great Patriotic War for the treatment of acute blood loss.
1 liter of solution contains: Na + - 138 mmol, K + - 2.7 mmol, Ca 2+ - 2.2 mmol, Mg 2+ - 0.4 mmol, C1 - 144 mmol, SO 4 2- - 0.4 mmol, HCO 3 - 1.6 mmol. Osmolarity 290 mOsm/l.
Saline infusin CIPC and solution LIPC-3 have not lost their value to date and can be used for losses of isotonic and hypertonic fluid.
Isotonic and isoionic solution (ionosteril - "Fresenius") includes ions in physiologically optimal ratio(1 l contains: Na + - 137 mmol, K + - 4 mmol, Ca 2+ - 1.65 mmol, Mg 2+ - 1.25 mmol, Cl - - 110 mmol, acetate - 36.8 mmol. Osmolarity of the solution 291 mOsm/l). It is used as a primary replacement solution for deficiency of plasma and extracellular fluid volume. Contraindicated in case of edema, hypertensive dehydration, severe renal failure.
Depending on the indications, a dose of 500-1000 ml or more per day is administered intravenously by drip at a rate of 3 ml/kg/h (70 drops/min at 70 kg body weight). In urgent cases, up to 500 ml in 15 minutes.
An isoionic solution of 5% or 10% glucose (fructose) is used for hypotonic dehydration and intravascular volume deficiency. Partially covers the need for carbohydrates. Contraindicated in hyperglycemia, overhydration, hypertensive dehydration and metabolic acidosis. The dose is determined specific situation. The rate of administration is 3 ml/kg body weight per hour.
Quartasol is an isotonic solution containing four salts (Na + - 124 mmol/l, K + - 20 mmol/l, Cl - - 101 mmol/l, HCO 3 - 12 mmol/l) and acetate - 31 mmol /l. Used as a replacement solution for polyion losses. Contraindicated in hyperkalemia, hypernatremia and hyperchloremia.
The daily dose is up to 1000 ml or more depending on the ionogram. The rate of administration is 3 ml/kg/h.
Lactasol is a physiological replacement solution with mild alkaline properties. Unlike isotonic sodium chloride solution, Ringer's solution has a balanced electrolyte composition, close to the composition of plasma.
1 liter of solution contains: Na + - 139.5 mmol, K + - 4 mmol, Ca 2+ - 1.5 mmol, Mg 2+ - 1 mmol, Cl - - 115 mmol, HCO 3 - 3.5 mmol, lactate - 30 mmol. Osmolarity 294.5 mOsm/L.
Lactasol and similar lactated Ringer's solution or Hartmann's solution are able to compensate for isotonic disturbances of hydroionic equilibrium. They are indicated to replace extracellular fluid deficiency with a balanced acid-base balance or mild acidosis. When added to colloidal solutions and erythrocyte mass, the rheological properties of the resulting mixtures are improved. As a result of the conversion of sodium lactate into bicarbonate in the body, an increase in bicarbonate buffer capacity and acidosis decreases. However positive properties lactasol as a corrector of water-electrolyte imbalances are realized only under conditions of aerobic glycolysis. In severe oxygen deficiency, lactasol can aggravate developing lactic acidosis.
The daily dose of lactasol and Ringer's lactate is up to 2500 ml. These solutions are administered intravenously at an average rate of 2.5 ml/kg/h, i.e. about 60 drops/min.
Lactasol and Ringer's lactate solution are contraindicated in cases of hypertensive overhydration, liver damage and lactic acidosis.
3. Basic solutions
Basic solutions include solutions of electrolytes and sugars that provide the daily need for water and electrolytes. These solutions must contain sufficient free water to replace electrolyte-free water losses during respiration and through the skin. At the same time, these solutions should provide the need for basic electrolytes or correct mild disturbances in the composition of electrolytes.
A basic solution with a high potassium content (Fresenius) contains electrolytes, a sufficient amount of free water and carbohydrates. It is a versatile alkaline electrolyte solution used to maintain water-electrolyte balance. It is indicated to meet the body's needs for water and electrolytes.
1 l contains: Na + - 49.1 mmol, K + - 24.9 mmol, Mg 2+ - 2.5 mmol, SG - 49.1 mmol, H 2 PO 4 - - 9.9 mmol, lactate - 20 mmol, sorbitol - 50 g. Calorie content 200 kcal/l. Osmolarity 430 mOsm/l.
This solution is contraindicated in case of shock, hyperkalemia, renal failure, water poisoning, sorbitol intolerance, methanol poisoning.
The solution is used as a continuous drip intravenous infusion. The rate of administration is 180 ml/h at 70 kg body weight. The average dose is 1500 ml/m2 body surface.
Semi-electrolyte solution with 5% glucose solution (Fresenius) provides the introduction of water and electrolytes with a small dose of carbohydrates. Used to cover water losses (hypertonic dehydration); loss of fluid poor in electrolytes; partial need for carbohydrates. Can be used as a carrier solution for electrolyte concentrates and medications compatible with the solution.
1 l contains: Na + - 68.5 mmol, K - 2 mmol, Ca 2+ - 0.62 mmol, Mg 2+ - 0.82 mmol, Cl - - 73.4 mmol, glucose monohydrate for injection - 55 g Osmolarity 423 mOsm/l.
Can be prescribed by intravenous continuous infusion up to 2000 ml/day at an average rate of 3 ml/kg body weight/hour.
Contraindicated in case of hyperglycemia, excess water in the body, hypotonic dehydration.
Electrolyte infusion solution (according to Hartig) provides the need for water and electrolytes. Designed to compensate for non-electrolyte water losses and mild electrolyte imbalances. 1 l contains: Na + - 45 mmol, K - 25 mmol, Mg 2+ - 2.5 mmol, Cl - - 45 mmol, acetate - 20 mmol, H 2 PO 4 - - 10 mmol. Osmolarity 150 mOsm/l.
The solution is contraindicated for hypotonic dehydration and overhydration, alkalosis, oliguria, shock.
The rate of administration is 3-4 ml/kg body weight/hour. The total dose is up to 1000-2000 ml/day. You should be careful not to overdose on water.
Glucose solution 5% is an isotonic electrolyte-free solution, 1 liter of which contains 950 ml of free water and 50 g of glucose. The latter is metabolized to form H 2 O and CO 2. 1 liter of solution gives 200 kcal. pH 3.0-5.5. Osmolarity 278 mOsm/l. Indicated for hypertensive dehydration, dehydration with a deficiency of free water. Base for adding other solutions. Contraindicated in case of hypotonic dehydration and overhydration, hyperglycemia, intolerance, methanol poisoning.
The dose is determined by the specific situation. The rate of administration is 4-8 ml/kg/hour. There is a danger of water poisoning!
Glucose solution 10% is a hypertonic electrolyte-free solution. Osmolarity 555 mOsm/l. 1 liter of solution gives 400 kcal. Indications and contraindications are the same as for a 5% glucose solution. The rate of administration is 2.5 ml/kg/h depending on the indications. There is a danger of water poisoning!
Isotonic sodium chloride solution, Ringer's solution, Ringer-Locke solution, lactasol and other isotonic and isoionic electrolyte solutions can be used as basic solutions. However, all these solutions cannot meet the body’s daily need for water. Therefore, they can be used together with electrolyte-free solutions of glucose or fructose, taking into account the basic needs for water and electrolytes.
A 5% fructose solution, like glucose solutions, is a donor of free water and energy (200 kcal/l). Indications for use are the same as for glucose solutions. Provides replacement of electrolyte-free water during fever, during surgery, 10% fructose solution is used especially widely in pediatrics. Contraindications, doses and rate of administration are the same as for glucose solutions.
4. Corrective solutions
Darrow's solution is a corrective solution used for potassium deficiency and alkalosis.
1 liter of Darrow solution (Fresenius) contains: Na + - 102.7 mmol, K + - 36.2 mmol, Cl - - 138.9 mmol. Osmolarity 278 mOsm/l.
Indications for its use: potassium deficiency, alkalosis resulting from loss of fluid containing potassium after administration of saluretics and corticosteroids.
Use up to 2000 ml per day in the form of a long-term drip intravenous infusion. The rate of administration is about 60 drops/min.
Contraindicated in hyperkalemia and renal failure.
Electrolyte solutions with 5% and 10% glucose solutions and high potassium content are used to replace potassium deficiency and correct alkalosis. These solutions are used for losses of potassium and chloride (for example, for losses of gastric juice).
1 liter of electrolyte solution with 5% glucose solution contains: Na + - 80 mmol, K + - 40 mmol, Cl - - 120 mmol, glucose monohydrate for injection - 55 g; 50 g glucose without crystallized water. Caloric content 200 kcal/l, osmolarity 517 mOsm/l. The same solution with a 10% glucose solution gives 400 kcal/l, its osmolarity is 795 mOsm/l.
The dosage is determined by the ionogram data. The rate of administration is 2.5 ml/kg/h. Due to the high concentration of potassium, the indicated rate of administration should not be exceeded! Maximum dose: 2000 ml/day for a body weight of 70 kg.
These solutions ("Fresenius") are contraindicated in case of acidosis, hyperkalemia, renal failure, excess water in the body and diabetes mellitus.
Chlosol is an isotonic solution enriched with potassium. The presence of sodium acetate allows the use of chlosol for the treatment of metabolic acidosis. This solution is indicated for hypokalemia without alkalosis, loss of sodium and chlorine.
1 liter of solution contains: Na + - 124 mmol, K + - 23 mmol, Cl - - 105 mmol; acetate - 42 mmol. Osmolarity 294 mOsm/l.
The dose is determined by the ionogram data. The rate of administration is 4-6 ml/kg/hour. The solution is contraindicated in hyperkalemia, metabolic alkalosis, overhydration and renal failure.
Ionocell (Fresenius) is an infusion solution for correcting intracellular loss of electrolytes potassium and magnesium aspartate.
Prescribed for combined potassium and magnesium deficiency. Can be used in the preoperative, intraoperative and postoperative periods for 2-5 days after major surgical interventions. This solution is indicated for paralytic obstruction, in the recovery phase after severe injuries and burns. It is also used after diabetic coma and acute myocardial infarction, and for heart rhythm disturbances.
1 liter of ionocell solution contains: Na + - 51.33 mmol, K + - 50 mmol, Mg 2+ - 25 mmol, Ca 2+ - 0.12 mmol, Zn 2+ - 0.073 mmol, Mn 2+ - 0.044 mmol, Co 2+ - 0.04 mmol, Cl - - 51.33 mmol, aspartate - 100.41 mmol. Osmolarity 558 mOsm/l.
Dosage in accordance with ionogram data. Intravenous continuous drip infusion of 1.5-2 ml/kg/h or maximum 2100 ml/day for a body weight of 70 kg. The rate of administration is 30-40 drops/min. Maximum up to 20 mmol potassium per hour.
Ionocell is contraindicated in severe renal failure, hyperkalemia, hypermagnesemia, intolerance to fructose and sorbitol, methanol poisoning, fructose-1,6-diphosphatase deficiency.
An isotonic sodium chloride solution containing excess chlorine, an acidic reaction, is used to correct hypochloremic alkalosis, especially in oliguria. It is indicated to compensate for the loss of gastric juice, but requires simultaneous administration of potassium.
Disol - a solution containing two salts: sodium chloride and sodium acetate. Indicated for the correction of hyperkalemic syndrome and hypotonic dehydration. The solution can be used for losses of sodium and chlorine and metabolic acidosis, in the initial period of oliguria caused by dehydration.
1 liter of solution contains: Na 2+ - 126 mmol, Cl - 103 mmol, acetate - 23 mmol. Osmolarity 252 mOsm/l.
Trisol - isotonic solution containing sodium chloride, potassium chloride and sodium bicarbonate. Used as a substitute for Ringer's solution, especially in metabolic acidosis.
1 liter of solution contains: Na + - 133 mmol, K + - 13 mmol, Cl - - 98 mmol, HCO 3 - 48 mmol. Osmolarity 292 mOsm/l.
Acesol- saline, relatively hypotonic solution containing sodium, potassium, chlorine and acetate. It is used to treat isotonic dehydration, with moderate changes in water and electrolyte balance. It has an alkalizing and anti-shock effect. Slow administration allows it to be used as a base solution.
1 liter of solution contains: Na + - 110 mmol, K + - 13 mmol, Cl - - 99 mmol, acetate - 24 mmol. Osmolarity 246 mOsm/l.
Literature
1. "Emergency Medical Care", edited by J.E. Tintinally, R. Crome, E. Ruiz, Translation from English by Dr. V.I. Kandrora, M.V. Neverova, A.V. . Suchkova, A.V. Nizovoy; edited by V.T. Bryusov;
2. Intensive therapy. Resuscitation. First aid: Textbook / Ed. V.D. Malysheva. - M.: Medicine. - 2000. - 464 p.: ill. - Textbook lit. For students of the postgraduate education system.
Similar documents
Classification and purpose of infusion solutions. Types and sources of colloidal infusion solutions, their chemical composition and components, areas of application in medicine, activity against blood diseases and various viral infections.
abstract, added 09/10/2009
Features of the pharmacological action and indications for the use of basic drug-substituting and detoxification solutions. Method of administration and dose. Side effects drugs and contraindications for use. Release form and storage conditions.
presentation, added 03/09/2014
General information about special cases of preparing solutions. Solutions of slowly soluble and coarsely crystalline substances. Obtaining easily soluble salts and complexes. Rules for the design of manufactured dosage forms. Preparation of phenol solutions.
abstract, added 05/11/2014
Methods of using solutions. Wet-dry dressings. Local baths and baths. Vegetable and mineral powders. Shake mixtures. Indications for the use of patches. Composition of masks. Gel as a dosage form. Aerosols, liniments, pastes.
presentation, added 02/24/2014
Dosage forms obtained by dissolving liquid, solid or gaseous substances in an appropriate solvent. Characteristics of non-aqueous solutions. Solubility medicines. Solvents used for the preparation of non-aqueous solutions.
abstract, added 10/30/2014
Solutions for internal use, manufactured by weight: prescription, manufacturing technology, quality control. Pharmacy technology for producing drops for oral administration. Improving solutions for internal use.
course work, added 11/28/2017
Injection forms, their characteristics. Advantages and disadvantages of injection administration. Classification, technology, requirements for injection solutions. Preparation of injection solutions without stabilizers, with a stabilizer, and physiological solutions.
course work, added 02/16/2010
Regulatory and technical documents regulating the requirements for the manufacture of the dosage form. Advantages and disadvantages of solutions, classification and types of solvents. Methods for obtaining purified water. Methods for prescribing solutions, their preparation.
course work, added 04/19/2015
The concept of pharmaceutical solutions, their classification. Solutions of solid and liquid substances. Theory of hydration and methods of fluid flow around particles. Concept and types of solvent. Technology of pharmaceutical solutions: aqueous, alcohol, glycerin, oil.
course work, added 08/21/2011
Blood substitutes as drugs (solutions) used for transfusion therapy. Functions of modern blood substitutes. The most common in medical practice. Composition, pharmacological action, indications for use of Ringer-Locke solution.