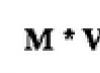One of the basic units in the International System of Units (SI) is The unit of quantity of a substance is the mole.
Mole – this is the amount of a substance that contains as many structural units of a given substance (molecules, atoms, ions, etc.) as there are carbon atoms contained in 0.012 kg (12 g) of a carbon isotope 12 WITH .
Considering that the value of the absolute atomic mass for carbon is equal to m(C) = 1.99 10 26 kg, the number of carbon atoms can be calculated N A, contained in 0.012 kg of carbon.
A mole of any substance contains the same number of particles of this substance (structural units). The number of structural units contained in a substance with an amount of one mole is 6.02 10 23 and is called Avogadro's number (N A ).
For example, one mole of copper contains 6.02 10 23 copper atoms (Cu), and one mole of hydrogen (H 2) contains 6.02 10 23 hydrogen molecules.
Molar mass(M) is the mass of a substance taken in an amount of 1 mole.
Molar mass is designated by the letter M and has the dimension [g/mol]. In physics they use the unit [kg/kmol].
In the general case, the numerical value of the molar mass of a substance numerically coincides with the value of its relative molecular (relative atomic) mass.
For example, the relative molecular weight of water is:
Мr(Н 2 О) = 2Аr (Н) + Аr (O) = 2∙1 + 16 = 18 a.m.u.
The molar mass of water has the same value, but is expressed in g/mol:
M (H 2 O) = 18 g/mol.
Thus, a mole of water containing 6.02 10 23 water molecules (respectively 2 6.02 10 23 hydrogen atoms and 6.02 10 23 oxygen atoms) has a mass of 18 grams. Water, with an amount of substance of 1 mole, contains 2 moles of hydrogen atoms and one mole of oxygen atoms.
1.3.4. The relationship between the mass of a substance and its quantity
Knowing the mass of a substance and its chemical formula, and therefore the value of its molar mass, you can determine the amount of the substance and, conversely, knowing the amount of the substance, you can determine its mass. For such calculations you should use the formulas:
where ν is the amount of substance, [mol]; m– mass of the substance, [g] or [kg]; M – molar mass of the substance, [g/mol] or [kg/kmol].
For example, to find the mass of sodium sulfate (Na 2 SO 4) in an amount of 5 moles, we find:
1) the value of the relative molecular mass of Na 2 SO 4, which is the sum of the rounded values of the relative atomic masses:
Мr(Na 2 SO 4) = 2Аr(Na) + Аr(S) + 4Аr(O) = 142,
2) a numerically equal value of the molar mass of the substance:
M(Na 2 SO 4) = 142 g/mol,
3) and, finally, the mass of 5 mol of sodium sulfate:
m = ν M = 5 mol · 142 g/mol = 710 g.
Answer: 710.
1.3.5. The relationship between the volume of a substance and its quantity
Under normal conditions (n.s.), i.e. at pressure R , equal to 101325 Pa (760 mm Hg), and temperature T, equal to 273.15 K (0 С), one mole of different gases and vapors occupies the same volume equal to 22.4 l.
The volume occupied by 1 mole of gas or vapor at ground level is called molar volumegas and has the dimension liter per mole.
V mol = 22.4 l/mol.
Knowing the amount of gaseous substance (ν ) And molar volume value (V mol) you can calculate its volume (V) under normal conditions:
V = ν V mol,
where ν is the amount of substance [mol]; V – volume of gaseous substance [l]; V mol = 22.4 l/mol.
And, conversely, knowing the volume ( V) of a gaseous substance under normal conditions, its quantity (ν) can be calculated :
A moth does not cause such negativity as a cockroach, but its presence is unpleasant. She is the one who can eat a single fur coat, spoil her favorite things, or gnaw on the harvest. Therefore, pest control is important, but first you need to find it.
Favorite places of moths
Prefers groceries. She eats everything without exception:
- grain;
- cereal;
- hot spices;
- flour;
- cookies;
- sugar;
- breadcrumbs and other products.
Each species has its own food preferences, but often food butterflies do not disdain anything. This means that a pest can be found in any dry products. The larva eats the product and spins a silk cocoon. Rolls and lumps form in flour and cereals; nests with worms can be found in nuts.
Food moths fly poorly. She is not able to move between rooms on her own, and ends up in the apartment with things or food.
What does a mole look like?
These are insects belonging to the order of butterflies. Appearance them directly depends on the species. Household pests are represented by the following types:
- food;
- wardrobe;
- grain;
- furniture butterfly.
The color is often inconspicuous - greenish, grayish. The sizes are small - up to 15 millimeters. An insect can place a nest in the house and in nature:
- rye;
- cabbage;
- apple
You can find out in more detail by reading the corresponding article on our website.

Detection of food butterfly
This type of moth caterpillar typically feeds on solid food:
- cabbage;
- leaves, stems;
- cereal;
- nuts.
In some cases, the adult does not have a mouthparts and lives for less than a week.
The nest should be looked for either in or near food. Often this is an apple tree, cereals, cabbage. First, a small nest is formed. It contains larvae.

Reasons for appearance
To infest an apartment with pests, a butterfly that is fertilized is enough. She flies into an apartment where there is comfortable conditions for reproduction. There are 3 main ways an insect can appear:
- passes from neighbors through cracks, entrance;
- flies into an open window or door;
- enters with things, purchases.
Signs of a pest
The presence of moths is indicated by the presence of eggs. But finding them is quite difficult because small sizes. In the closet, if you look carefully, you can see a large number of threads of cobwebs and pupae.
Flying insects usually do not go unnoticed. If there are 2 or more individuals in the apartment, you should immediately arm yourself with a flashlight and begin inspecting the premises. Check thoroughly in places where cereals are stored, in things made from natural fabrics, and in kitchen furniture.

How to protect your home
It is important to know how to protect yourself from moths. Prevention will help protect your apartment from pests. The following measures are worth noting:
- use of special repellents;
- checking all food products after purchase;
- purchasing only those foods that can be eaten during the week;
- You should not purchase products in places where the products are sold at a very low price.
For moths, there are no more dangerous herbs with a strong odor:
- rosemary;
- sagebrush;
- lavender;
- tansy.
You can also use:
- horse chestnut;
- vinegar;
- orange peel;
- strawberry or laundry soap.
How to get rid of food moths (video)
If you put a jar filled with semolina in the cupboard with lavender, the larvae will survive, but you should be afraid explosive mixture there will only be butterflies. Preventive measures It is recommended to conduct it comprehensively.
At times, even in a perfectly clean apartment, moths are found. The owners are perplexed where she comes from. There is a feeling that insects appear out of nowhere. Harmful guests spoil property and all kinds of products.
Information about finding moths in an apartment contains the following sections:
How do moths appear in an apartment?
When considering the circumstances of the appearance of harmful insects in an apartment, it is useful to know that they are divided into 2 types:
- Clothes.
- Food.
Although they are very similar in appearance, they appear for different reasons.
The emergence of food moths
Homeowners are often tormented by the question: “Can moths grow in a dirty room?” The answer is no. The appearance of insects is absolutely not affected by dirt or non-compliance sanitary standards in the apartment. Moths spread in places where you can eat delicious food. In addition, we should not forget that a food lover eats almost everything that comes his way.
Reasons why food moths appear:
- It enters the apartment in products that were infested with these pests in a store or warehouse.
- Flies through ventilation holes from neighbors.
- Flies into the apartment through window or doorways.
Quite often, in stores or in supplier warehouses, products are stored in violation of sanitary standards. In this regard, you need to be careful with promotional products or those sold at a discount.
If a buyer purchases nuts, dried fruits, spices, dry pet food, flour, seeds, cereals, and roots at a promotion, there is a high probability that voracious pests will enter the apartment. This reason is the most common.
Moth entry through ventilation holes also needs to be taken into account. Therefore, it is advisable to cover these holes with gauze or fine mesh.
Moths don't fly well. Therefore, entry into the apartment through window or doorways is unlikely.
Appearance of clothes moth
This type of moth is very interested in natural products, mainly fur and wool. And it doesn’t matter how clean things are. The main thing is that you have them. Reasons for clothes moths entering an apartment:- Moths enter homes with contaminated products.
- Insects are brought in by domestic animals.
The source of the appearance of pupae and larvae of the pest is most often purchased items, both new and used. Even buying clothes in an expensive store does not guarantee the absence of unpleasant individuals.
Having entered the apartment, the voracious pest begins to actively reproduce. As a result, the premises have damaged carpets, furniture, fur products, and woolen clothing.
Not new material much more accessible for moths to feed on. Therefore, you need to thoroughly check all wardrobe and interior items for the presence of pest larvae.
Clothes moths can also be “visited” by dogs of the following breeds:
- Wire-haired dachshund.
- Bobtail.
- South Russian Shepherd Dog.
- Giant Schnauzer.
Shoes, clothes and carpets also need to be looked after, especially:
- Furniture with natural upholstery.
- Knitted clothes.
- Natural wool carpets.
- Outerwear. Mostly moths spoil products made of wool and fur.
- Shoes with natural fur.
How to find moths in an apartment?
Often, the presence of insects is indicated by moths flying around the apartment. If they are noticed, this indicates the presence of larvae in things and products. They managed to complete their life cycle and turned into butterflies. They say you should look for them depending on the species in the appropriate places. 
Moth eggs are not that easy to spot. Although it is possible to see pupated gluttons and caterpillar larvae in the cereals. Holes appear on woolen items, and fur products specific paths and sand (larval excrement) are visible.
Search for food moths
This individual lives in the kitchen. It lives in all kinds of uncovered or loosely tied loose cereals. In addition, larvae can often be found in nuts, tea and dried fruits.
In order to detect a voracious insect, you need to carefully examine the contents of all containers and bags of food in the apartment. When larvae are found, you should get rid of products with them. It is also necessary to treat the surfaces where these products were stored. To do this you will need a sponge and cleaning agent.
Moths can also occur in poorly ventilated areas with high humidity. Therefore, the apartment must be systematically ventilated. And if pests have already settled in the kitchen, then ventilation should be done more often.
To avoid reappearance food moth, you need to hide the cereals in an impenetrable container or in tied plastic bags, but so that the pest larvae cannot crawl into them.
Searching for clothes moths
Many people mistakenly believe that clothes moths feed on all kinds of clothing. But this is not true. These gluttons do not eat clothes, but pieces of human skin left on them after wearing them. That is, voracious pests can settle not only in a fur coat made of natural fur, but also in pants, sweaters, hats, etc.
You need to start looking for moths from the closet. This is the place she likes to live most. First of all, you should explore all the cracks in search of larvae and butterflies. The closet should be completely emptied of clothes, which should be thoroughly cleaned.
Advice!
Hang your clothes outside for a couple of days so that they receive direct sunlight. Moths don't like this!
To get rid of larvae, you need to apply disinfectants, which contain dichlorvos.
After the cabinet, all cabinet furniture in the room should be inspected and processed. Then the moth will not return.
What do moths eat?
The domestic animal causes a lot of inconvenience indoors in the form of damaged things, products, and property.
Favorite delicacies of food moths
These insects are divided into several types. Therefore, they eat foods made from grains and flour. Another group of individuals loves dried fruits, sweets and confectionery products.
The range of products that this type of moth prefers:
- bread,
- dried fruits,
- pasta,
- chocolate,
- flour,
- flour pastries,
- cookie,
- nuts,
- candies,
- cereal,
- seeds,
- cereals.
Taste preferences of the duffel moth
This pest is divided into clothing, fur, wool, carpet, and furniture moths.
List of dungeon moth treats:
- knitwear,
- blankets,
- socks,
- products made from bird feathers,
- knitted hats,
- furniture upholstery,
- mittens,
- sheepskin coats,
- fur shoes,
- natural fur coats,
- felt slippers,
- felt boots,
- wool sweaters,
- carpets.
This is not the whole list, because the insect eats everything that consists of natural fiber. The pest can even ruin synthetic linen if it contains cotton or wool.  Clothing that has been worn for a long time is also subject to attack if it contains particles of epidermis from human skin.
Clothing that has been worn for a long time is also subject to attack if it contains particles of epidermis from human skin.
On a note!
Do not hide clothes in plastic bags. If a moth has chosen a thing, it will gnaw even such material!
Yesterday I promised to explain this accessible language. This is important for understanding chemistry. If you understand it once, you won’t forget it later.
Chemistry has its own language, like any science. 2H 2 + O 2 → 2H 2 O - in chemical language, a recording of the reaction of the formation of water from simple substances, hydrogen (H) and oxygen (O). Small numbers refer to the number of atoms (They come after the symbol chemical element), large ones - to the number of molecules. From the equation it is clear that two hydrogen molecules combine with one oxygen molecule and as a result comes out two water molecules. Attention - this is very important to understand! It is precisely molecules that connect with molecules, not “gram with gram,” but molecule with molecule.
This proportion will always remain:
Everything would be fine, but there are two problems. The first one is in real life we cannot measure one million molecules of oxygen or hydrogen. We will be able to measure one gram or one ton of reagents. Second, the molecules are very small. There are 6.7 x 10 24 of them in one glass of water. Or, in the usual notation, 6.7 trillion trillion (that’s right - almost seven trillion times a trillion molecules). It is inconvenient to operate with such numbers.
What is the way out? Molecules also have mass, albeit very small. We just take mass of one molecule, multiply by number of molecules and we get the mass we need. We agreed this way - we take a very large number of molecules (600 billion trillion pieces) and invent for this quantity special unit of measurement mole. Like for 12 pieces of something there is a special name "dozen", and when they talk about “ten dozen”, they mean 120 pieces. 5 dozen eggs = 60 pieces. Same with moles. 1 mole is 600 billion trillion molecules or, in mathematical notation, 6.02 10 23 molecules. That is, when we are told “1 mole” of hydrogen, we know that we are talking about 600 billion trillion molecules of hydrogen. When they talk about 0.2 moles of water, we understand that we are talking about 120 billion trillion water molecules.
Once again - the moth is just like that counting unit, only specifically for molecules. Like “ten”, “dozen” or “million”, only much more.
Continuing the table above, you can write:
We solved the first problem; writing 1 mole or 2 moles is much more convenient than 600 billion trillion molecules or 1.2 trillion trillion molecules. But for convenience alone, there was no need to fence the garden. The second problem, as we remember, is the transition from number of molecules(don’t count them individually!) to mass of matter, to the fact that we can measure on scales. This number of molecules in one mole (it is a little strange, non-round - 6.02·10 23 molecules) was chosen for a reason. One mole of carbon molecules weighs exactly 12 grams.
It is clear that all molecules are different. There are large and heavy ones - they may contain many atoms, or not very many, but the atoms themselves are heavy. And there are small and light molecules. For each atom and for many molecules there are tables with their molar mass. That is, with the weight of one mole of such molecules (if not, you can easily calculate it yourself by adding up the molar masses of all the atoms that make up the molecule). Molar mass is measured in grams/mol (how many grams one mole weighs, that is, how many grams 6.02·10 23 molecules weigh). We remember that a mole is just a counting unit. Well, as if the directory wrote - 1 dozen chicken eggs weighs 600 grams, and 1 dozen ostriches weighs 19 kilograms. A dozen is just a quantity (12 pieces), and the eggs themselves, chicken or ostrich, weigh differently. And a dozen of these or other eggs also weigh differently.
Same with molecules. 1 mole of small and light hydrogen molecules weighs 2 grams, and 1 mole of large sulfuric acid molecules weighs 98 grams. 1 mole of oxygen weighs 32 grams, 1 mole of water weighs 18 grams. Here is a picture as an example, where small hydrogen molecules and large oxygen molecules are visible. This picture is a graphical representation of the reaction 2H 2 + O 2 → 2H 2 O.
We continue to fill out the table:
Do you see the transition from number of molecules to their mass? Do you see that the law of conservation of matter is fulfilled? 4 grams + 32 grams gives 36 grams.
Now we can decide simple tasks in chemistry. Here is the most primitive one: There were 100 oxygen molecules and 100 hydrogen molecules. What will happen as a result of the reaction? We know that for 1 molecule of oxygen you need 2 molecules of hydrogen. Therefore, all 100 hydrogen molecules will react (and 100 water molecules will be formed), but not all oxygen will react, another 50 molecules will remain. Oxygen is in excess.
As I said above, no one counts molecules as pieces. Substances are usually measured in grams. Now a problem from a school textbook: there are 10 g of hydrogen and 64 g of oxygen, what happens if you mix them? First, we must convert the masses into moles (that is, into the number of molecules or the amount of substance, as chemists say). 10 g of hydrogen is 5 moles of hydrogen (1 mole of hydrogen weighs 2 grams). 64 g of oxygen is 2 moles (1 mole weighs 32 grams). We know that for 1 mole of oxygen, 2 moles of hydrogen are lost during the reaction. This means that in our case, all the oxygen (2 moles) and 4 moles of hydrogen out of five will react. This results in 4 moles of water and one mole of hydrogen remaining.
Let's convert the answer back into grams. All oxygen (64 grams) and 8 grams of hydrogen (4 mol * 2 g/mol) will react. 1 mole of hydrogen will remain unreacted (that's 2 grams) and you'll get 72 grams of water (4 moles * 18 g/mol). The law of conservation of matter is again fulfilled - 64 + 10 = 72 + 2.
I think that by now it should be clear to everyone. 1 mole is simply the number of molecules. Molar mass is the mass of one mole. It is needed in order to move from the mass of matter (with which we work in real world) to the number of molecules, or the amount of substance needed for reactions.
We repeat again:
a) substances react in the ratio of n molecules of one to m molecules of the other. This proportion will be the same for 100 molecules of the original substance, and for one hundred trillion, or for one hundred trillion trillion.
b) for convenience, in order not to count molecules as pieces, they came up with a special counting unit - the mole, that is, 6.02·10 23 molecules at once. The number of these moles is called the usual “amount of substance”
c) a mole of each substance weighs differently, because The molecules and atoms that make up a substance themselves weigh differently. The mass of one mole of a substance is called its molar mass. Another example is ordinary and sand-lime bricks weigh differently. If we draw an analogy, then the “weight of a thousand bricks” is the “molar mass” (with the difference that there are not 1000 molecules, but more). The mass of this “thousand bricks” is different for sand-lime bricks and ordinary bricks.
d) we fence this whole garden so that it is easy to move from the mass of reagents to the amount of substance (number of molecules, number of moles) and back. And you need to go back and forth because in the real world we measure reagents in grams, and chemical reactions are proportional not to mass, but to the number of molecules.
P.S. For chemists and others, I have intentionally simplified a lot here. I don’t need to explain that 12 grams weighs not 1 mole of carbon, but 1 mole of molecules of the C 12 isotope, or that instead of “molecules” it would be necessary to write “structural units” (molecules, ions, atoms...), not specifically mentioned that 1 mole of gas occupies the same volume under the same conditions and much more
What I didn’t like in the textbooks was only a formal definition of mole, without indicating the meaning of this concept and what it is needed for.
In chemistry the concept “ mole" This is such a quantity substances, which contains approximately 6.02214*10^23 of its elementary particles - molecules, ions or atoms. To make calculations easier, this huge number, called Avogadro's number, is often rounded to 6.022*10^23. A mole is measured in grams.
Instructions
To find mole substances, you need to remember a very simple rule: the mass of one mole of any substances is numerically equal to its molecular weight, only expressed in other quantities. How is molecular weight determined? Using the periodic table, you will find out the atomic mass of each element that makes up the molecule substances. Next, you need to add the atomic masses, taking into account the index of each element, and you will get the answer.
For example, widely used in agriculture fertilizer, ammonium nitrate (or otherwise ammonium nitrate). The formula for this substances NH4NO3. How to determine what it is equal to mole? First of all, write down the empirical (that is, general) formula substances: N2H4O3.
Calculate its molecular weight taking into account the index of each element: 12*2 + 1*4 + 16*3 = 76 amu. (atomic mass units). Therefore, its molar mass (that is, the mass of one mole) is also 76, only its dimension is: gram/ mole. Answer: one mole ammonium nitrate weighs 76 grams.
Suppose you are given such a task. It is known that the mass of 179.2 liters of some gas is 352 grams. It is necessary to determine how much one weighs mole this gas. It is known that under normal conditions one mole any gas or mixture of gases occupies a volume of approximately 22.4 liters. And you have 179.2 liters. Do the calculation: 179.2/22.4 = 8. Therefore, this volume contains 8 moles of gas.
Dividing the mass known from the conditions of the problem by the number of moles, you get: 352/8 = 44. Therefore, one mole of this gas weighs 44 grams - this is carbon dioxide, CO2.
If there is a certain amount of gas of mass M, enclosed in a volume V at a given temperature T and pressure P. It is required to determine its molar mass (that is, find what its mole). The universal Mendeleev-Clapeyron equation will help you solve the problem: PV = MRT/m, where m is the very molar mass that we need to determine, and R is the universal gas constant equal to 8.31. Transforming the equation, you get: m = MRT/PV. By substituting known quantities into the formula, you will find what is equal to mole gas.
Various formulas will help you find the amount of a substance whose unit of measurement is mole. Also, the amount of a substance can be found using the reaction equation given in the problem.

Instructions
Having the mass and name of the substance, you can easily find the amount of the substance: n = m/M, where n is the amount of the substance ( mole), m is the mass of the substance (g), M is the molar mass of the substance (g/ mole). For example, the mass of sodium chloride is 11.7 g, find the amount of the substance. To substitute the required values into the formula, you need to find the molar mass of sodium chloride: M(NaCl) = 23+35.5 = 58.5 g/ mole. Substitute: n(NaCl) = 11.7/58.5 = 0.2 mole.
If we are talking about gases, then the following formula applies: n = V/Vm, where n is the amount of substance( mole), V - volume of gas (l), Vm - molar volume of gas. Under normal conditions (pressure 101,325 Pa and temperature 273 K), the molar volume of the gas is constant and equal to 22.4 l/ mole. For example, what amount of substance will a 30 liter volume of nitrogen have under normal conditions? n(N2) = 30/22.4 = 1.34 mole.
Another formula: n = N/NA, where n is the amount of substance( mole), N is the number of molecules, NA is Avogadro’s constant, equal to 6.02*10 to the 23rd power (1/ mole).For example, what amount of substance is contained in 1.204 * 10 to the 23rd power? We solve: n = 1.204*10 to the 23rd power/6.02*10 to the 23rd power = 0.2 mole.
Using any reaction equation, you can find the amount of substances that entered into the reaction and were formed as a result of it. 2AgNO3 + Na2S = Ag2S + 2NaNO3. From this equation it is clear that 2 mole silver nitrate reacted with 1 mole sodium sulfide, resulting in the formation of 1 mole silver sulfide and 2 mole sodium nitrate. Using these quantities of substances, you can find other quantities required in the problems. Let's look at an example.
A solution containing sodium sulfide was added to a solution containing silver nitrate weighing 25.5 g. What amount of silver sulfide is formed in this case?
First, we find the amount of silver nitrate substance by first calculating its molar mass. M(AgNO3) = 170 g/ mole. n(AgNO3) = 25.5/170 = 0.15 mole. The reaction equation for this problem is written above, it follows that from 2 mole silver nitrate is formed 1 mole silver sulfide. Determine how much mole silver sulfide is formed from 0.15 mole silver nitrate: n(Ag2S) = 0.15*1/2 = 0.075 mole.