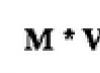Description:
Wetting a copper plate in hydrochloric acid and bringing it to the burner flame, we notice an interesting effect - coloring of the flame. The fire shimmers with beautiful blue-green shades. The spectacle is quite impressive and mesmerizing.
Copper gives the flame green tint. With a high copper content in the combustible substance, the flame would have a bright green color. Copper oxides give an emerald green color. For example, as can be seen from the video, when wetting copper hydrochloric acid the flame turns blue with a greenish tint. And calcined copper-containing compounds soaked in acid color the flame azure blue.
For reference: Barium, molybdenum, phosphorus, and antimony also give green color and its shades to fire.
Explanation:
Why is the flame visible? Or what determines its brightness?
Some flames are almost invisible, while others, on the contrary, shine very brightly. For example, hydrogen burns with an almost completely colorless flame; the flame of pure alcohol also shines very weakly, but a candle and a kerosene lamp burn with a bright luminous flame.
The fact is that the greater or lesser brightness of any flame depends on the presence of hot solid particles in it.
Fuel contains carbon in greater or lesser quantities. Carbon particles become heated before they burn, which is why the flame gas burner, a kerosene lamp and a candle shines - because it is illuminated by hot carbon particles.
Thus, it is possible to make a non-luminous or weakly luminous flame bright by enriching it with carbon or heating non-combustible substances with it.
How to get different colored flame?
To obtain a colored flame, not carbon is added to the burning substance, but metal salts that color the flame in one color or another.
The standard method of coloring a faintly luminous gas flame is to introduce metal compounds into it in the form of highly volatile salts - usually nitrates (salt nitric acid) or chlorides (salts of hydrochloric acid):
yellow- sodium salts,
red - strontium, calcium salts,
green - cesium salts (or boron, in the form of boronethyl or boronmethyl ether),
blue - copper salts (in the form of chloride).
IN Selenium colors the flame blue, and boron colors the flame blue-green.
This ability of burning metals and their volatile salts to impart a certain color to a colorless flame is used to produce colored lights (for example, in pyrotechnics).
What determines the color of a flame (in scientific language)
The color of a fire is determined by the temperature of the flame and what chemicals it burns. The high temperature of the flame allows atoms to jump for some time to a higher temperature. energy state. When the atoms return to their original state, they emit light at a specific wavelength. It corresponds to the structure of the electronic shells of a given element.
Page 1
The yellow color of the flame is due to N3 atoms (X 0 589 μm), white is due to the presence of BaO and M § O.
Adding sodium nitrate salt crystal to the flame causes the flame to appear yellow.
The method is very sensitive: the opening minimum is 0.0001 y - Therefore, the presence of sodium can only be judged if yellow The flame will be bright and does not disappear for 10 - 15 seconds.
Ignition of the gas generator is completed when the test tap at exhaust pipe the gas will burn steadily even flame purple with a pink tint. A yellow flame indicates poor gas quality, and red, slightly smoky flames are a sign of the presence of tar in the gas. If the quality of the gas is satisfactory, it contains less than 0 5 - 0 6% oxygen. If the gas does not burn at all or flares up and goes out, this indicates low temperature in the core; it is necessary to ignite the gas generator more strongly.
This kind of conclusion is not flawless. Firstly, the yellow color of the flame can veil the color of the flame caused by other elements, and secondly, the yellow color can be caused by impurities of sodium compounds contained in the main substance being determined.
The method is very sensitive: the opening minimum is 0.0001 mcg. Therefore, the presence of sodium can be concluded only if the yellow color of the flame is bright and does not disappear within 10 - 15 seconds.
To clean the wires, they are supplied with borax pearls, which are heated as shown in Fig. 2, a, only on one side; in this case, the ball moves in the opposite direction along the platinum wire and dissolves all contaminants of the latter. After repeating this technique three times, the wire will be cleared of everything foreign, with the exception of an insignificant amount of glass adhering to it, which in turn can be removed if the wire is calcined in the part of the flame with the highest temperature until the yellow color of the sodium flame completely disappears.
The yellow color of the flame, caused by minute impurities of sodium salts, often masks purple flame potassium In this case, the flame should be viewed through a glass prism containing an indigo solution, which absorbs the yellow part of the spectrum.
The ionization potentials (energies) of alkali and alkaline earth metals are very small, therefore, when a metal or its compound is introduced into a burner flame, the element is easily ionized, coloring the flame in a color corresponding to its spectral line of excitation. The yellow color of the flame is characteristic of sodium compounds, violet - for potassium compounds, brick red - for calcium compounds.
Why then does iron wire give the same light? By carefully cleaning the surface of the iron wire, you can show that the yellow color of the flame is not due to the iron; The yellow color is due to the presence of small amounts of salt on the surface of the iron wire, grasped with fingers, which always have traces of salt on them. A yellow flame is a very sensitive test for the presence of sodium. The eye may notice a change in the color of the flame resulting from the introduction of an element into the flame in an amount significantly less than 1 microgram. Detecting such a small amount of a substance without this flame method is far from an easy task for a chemist.
| Part of a diagram of the energy levels of the valence electrons of the sodium atom. The terma symbol is a digital representation of different energy levels. The numbers on the lines indicate the corresponding wavelengths in nanometers. |
In Fig. 2 - 1, in accordance with generally accepted concepts, shows some energy levels of the outer electrons of a neutral sodium atom. The excited electron tends to return to its normal (3s) state; upon returning to normal, a photon is emitted. The emitted photon has a certain amount of energy determined by the location of the energy level. In the example given, the emitted radiation produces the familiar yellow color of the sodium flame and sodium lamp.
Pages: 1
In laboratory conditions, it is possible to achieve a colorless fire, which can be determined only by the vibration of the air in the combustion area. Household fire is always “colored”. The color of a fire is determined primarily by the temperature of the flame and what chemicals it burns. The high temperature of the flame allows atoms to jump to a higher energy state for some time. When the atoms return to their original state, they emit light at a specific wavelength. It corresponds to the structure of the electronic shells of a given element.
Famous blue a flame that can be seen when burning natural gas, is caused by carbon monoxide, which gives this shade. Carbon monoxide, a molecule made up of one oxygen atom and one carbon atom, is a byproduct of the combustion of natural gas.
Try sprinkling on the burner gas stove a little table salt - yellow tongues will appear in the flame. This yellow-orange flame give sodium salts (a salt, remember, this is sodium chloride). Wood is rich in such salts, which is why an ordinary forest fire or household matches burn yellow flame.
Copper gives the flame green shade. With a high copper content in the combustible substance, the flame has a bright green color, almost identical to white.
Barium, molybdenum, phosphorus, and antimony also give green color and its shades to fire. IN blue Selenium colors the flame, and in blue-green- boron A red flame will give lithium, strontium and calcium, a purple flame will give potassium, and a yellow-orange hue will come out when sodium is burned.
Flame temperature when burning certain substances:
Did you know...
Due to the ability of atoms and molecules to emit light a certain color a method was developed for determining the composition of substances, which is called spectral analysis. Scientists study the spectrum that a substance emits, for example, when it burns, compare it with the spectra of known elements, and thus determine its composition.
Light a candle and carefully examine the flame. You will notice that it is not uniform in color. The flame has three zones (Fig.). Dark zone 1 is at the bottom of the flame. This is the most cold zone compared to others. The dark zone is bordered by the brightest part of the flame 2. The temperature here is higher than in the dark zone, but the most heat at the top of the flame 3.
To ensure that different flame zones have different temperatures, you can conduct such an experiment. Place a splinter (or match) into the flame so that it crosses all three zones. You will see that the splinter is more charred where it hits zones 2 and 3. This means that the flame is hotter there.
To all the answers I will add one more detail that is used by chemists. There are several zones in the flame structure. The inner, blue, coldest (relative to other zones) is the so-called restorative flame. Those. reduction reactions can be carried out in it (for example, metal oxides). The upper part, yellow-red, is the hottest zone, also called the oxidizing flame. It is here that the oxidation of substance vapors with atmospheric oxygen occurs (if, of course, we are talking about an ordinary flame). It is possible to carry out appropriate chemical reactions in it.
The color of the fire depends on chemical elements which burn during combustion, for example, if you want to see a blue light, then it appears when natural gas burns, and is caused by carbon monoxide, which gives this tint. Yellow flames appear when sodium salts decompose. Wood is rich in such salts, so an ordinary forest fire or household matches burn with a yellow flame. Copper gives the flame a green tint. With a high copper content in the combustible substance, the flame has a bright green color, almost identical to white.
Barium, molybdenum, phosphorus, and antimony also give green color and its shades to fire. Selenium colors the flame blue, and boron colors the flame blue-green. The red flame will give lithium, strontium and calcium, purple potassium, a yellow-orange tint comes out when sodium burns.
Well, if anyone is interested more detailed information please visit this page http://allforchildren.ru/why/misc33.php
The color of the flame depends on its temperature, as well as on the composition of the substance that burns:
4300K - white-yellow, the brightest light;
5000K - cool white color;
6000K - white with light blue
8000K - blue-blue - the lighting quality is worse.
12000K purple
So, in fact, the hottest flame of a candle is from the bottom, and not from the top, as Maxim26ru 325 said, and the temperature at the tip of the flame is higher only due to the presence of gravity on Earth - convection currents arise, as a result of which the heat rushes vertically upward.
The color of fire depends directly on the temperature of the flame, and the temperature, in turn, releases a substance that will give a certain color in its spectrum. For example:
Carbohydrate dates are blue in color;
Boron - Blue-green;
Sodium salts give off yellow-orange color
Green color comes from the release of copper, molybdenum, phosphorus, barium, antimony
Blue is selenium
Red from excretion of lithium and calcium
Purple date potassium
At first, as Alexander Antipov said, yes, the color of the flame is determined by its temperature (if I’m not mistaken, it was proven by Planck). And then the material of what is burning accumulates in the flame. Atoms different elements are able to absorb quanta with a certain energy and emit them back, but with an energy that depends on the nature of the atom. Yellow is the color of sodium in the flame. Sodium is found in any natural organic material. And yellow color can drown out other colors - this is a feature of human vision.
Well, it depends what kind of fire it is. It can be any color, depending on the burning substance. And this blue-yellow flame is from its heating. The further the flame is from the burning substance, the more oxygen there is. And the more oxygen, the hotter the flame and therefore lighter and brighter.
In general, the temperature inside the flame is different and changes over time (depending on the influx of oxygen and combustible substance). Blue color means that the temperature is very high up to 1400 C, yellow - the temperature is slightly lower than when blue flame.
The color of the flame may vary depending on chemical impurities.
During the combustion process, a flame is formed, the structure of which is determined by the reacting substances. Its structure is divided into areas depending on temperature indicators.
Definition
Flame refers to gases in hot form, in which plasma components or substances are present in solid dispersed form. They carry out transformations of physical and chemical type, accompanied by glow, release of thermal energy and heating.
The presence of ionic and radical particles in a gaseous medium characterizes its electrical conductivity and special behavior in an electromagnetic field.
What are flames
This is usually the name given to processes associated with combustion. Compared to air, gas density is lower, but high temperatures cause gas to rise. This is how flames are formed, which can be long or short. Happens often smooth transition one form into another.
Flame: structure and structure
For determining appearance For the described phenomenon, it is enough to ignite. The non-luminous flame that appears cannot be called homogeneous. Visually, three main areas can be distinguished. By the way, studying the structure of the flame shows that various substances burn with education various types torch.
When a mixture of gas and air burns, a short flame is first formed, the color of which is blue and purple shades. The core is visible in it - green-blue, reminiscent of a cone. Let's consider this flame. Its structure is divided into three zones:
- A preparatory area is identified in which the mixture of gas and air is heated as it exits the burner opening.
- This is followed by the zone in which combustion occurs. It occupies the top of the cone.
- When there is a deficiency air flow, the gas does not burn completely. Carbon divalent oxide and hydrogen residues are released. Their combustion takes place in the third region, where there is oxygen access.
Now let's look separately different processes combustion.
Burning candle
Burning a candle is similar to burning a match or lighter. And the structure of a candle flame resembles a hot gas stream, which is pulled upward due to buoyancy forces. The process begins with heating the wick, followed by evaporation of the wax.
The lowest zone, located inside and adjacent to the thread, is called the first region. It has a slight glow due to large quantity fuel, but a small volume of oxygen mixture. Here, the process of incomplete combustion of substances occurs, releasing which is subsequently oxidized.

The first zone is surrounded by a luminous second shell, which characterizes the structure of the candle flame. A larger volume of oxygen enters it, which causes the continuation of the oxidation reaction with the participation of fuel molecules. Temperatures here will be higher than in the dark zone, but not sufficient for final decomposition. It is in the first two areas that high heat Droplets of unburned fuel and coal particles produce a luminous effect.
The second zone is surrounded by a low-visibility shell with high temperature values. Many oxygen molecules enter it, which contributes to the complete combustion of fuel particles. After the oxidation of substances, the luminous effect is not observed in the third zone.
Schematic illustration
For clarity, we present to your attention an image of a burning candle. Flame circuit includes:
- The first or dark area.
- Second luminous zone.
- The third transparent shell.
The candle thread does not burn, but only charring of the bent end occurs.

Burning alcohol lamp
For chemical experiments Small containers of alcohol are often used. They are called alcohol lamps. The burner wick is soaked with the liquid poured through the hole. liquid fuel. This is facilitated by capillary pressure. When the free top of the wick is reached, the alcohol begins to evaporate. In the vapor state, it is ignited and burns at a temperature of no more than 900 °C.
The flame of an alcohol lamp has a normal shape, it is almost colorless, with a slight tint of blue. Its zones are not as clearly visible as those of a candle.
Named after the scientist Barthel, the beginning of the fire is located above the burner grid. This deepening of the flame leads to a decrease in the inner dark cone, and the middle section, which is considered the hottest, emerges from the hole.

Color characteristics
Various radiations are caused by electronic transitions. They are also called thermal. Thus, as a result of combustion of a hydrocarbon component in air, a blue flame is caused by the release H-C connections. And when C-C particles are emitted, the torch turns orange-red.
It is difficult to consider the structure of a flame, the chemistry of which includes compounds of water, carbon dioxide and carbon monoxide, and the OH bond. Its tongues are practically colorless, since the above particles, when burned, emit radiation in the ultraviolet and infrared spectrum.
The color of the flame is interconnected with temperature indicators, with the presence of ionic particles in it, which belong to a certain emission or optical spectrum. Thus, the combustion of certain elements leads to a change in the color of the fire in the burner. Differences in the color of the torch are associated with the arrangement of elements in different groups periodic system.
Fire is examined with a spectroscope for the presence of radiation in the visible spectrum. At the same time, it was found that simple substances from the general subgroup also cause a similar coloration of the flame. For clarity, sodium combustion is used as a test for this metal. When brought into the flame, the tongues turn bright yellow. Based color characteristics highlight the sodium line in the emission spectrum.
It is characterized by the property of rapid excitation of light radiation from atomic particles. When non-volatile compounds of such elements are introduced into the fire of a Bunsen burner, it becomes colored.
Spectroscopic examination shows characteristic lines in the area visible to the human eye. The speed of excitation of light radiation and the simple spectral structure are closely related to the high electropositive characteristics of these metals.
Characteristic
The flame classification is based on the following characteristics:
- aggregate state of burning compounds. They come in gaseous, airborne, solid and liquid forms;
- type of radiation, which can be colorless, luminous and colored;
- distribution speed. There is fast and slow spread;
- flame height. The structure can be short or long;
- nature of movement of reacting mixtures. There are pulsating, laminar, turbulent movement;
- visual perception. Substances burn with the release of a smoky, colored or transparent flame;
- temperature indicator. The flame can be low temperature, cold and high temperature.
- state of the fuel - oxidizing reagent phase.
Combustion occurs as a result of diffusion or pre-mixing of the active components.

Oxidative and reduction region
The oxidation process occurs in a barely noticeable zone. It is the hottest and is located at the top. In it, fuel particles undergo complete combustion. And the presence of oxygen excess and combustible deficiency leads to an intense oxidation process. This feature should be used when heating objects over the burner. That is why the substance is immersed in top part flame. This combustion proceeds much faster.
Reduction reactions take place in the central and lower parts of the flame. Contained here large stock flammable substances and a small amount of O 2 molecules that carry out combustion. When introduced into these areas, the O element is eliminated.
As an example reduction flame use the process of splitting ferrous sulfate. When FeSO 4 enters the central part of the burner torch, it first heats up and then decomposes into ferric oxide, anhydride and sulfur dioxide. In this reaction, reduction of S with a charge of +6 to +4 is observed.
Welding flame
This type of fire is formed as a result of the combustion of a mixture of gas or liquid vapor with oxygen from clean air.

An example is the formation of an oxyacetylene flame. It distinguishes:
- core zone;
- middle recovery area;
- flare extreme zone.
This is how many gas-oxygen mixtures burn. Differences in the ratio of acetylene and oxidizing agent lead to different types flame. It can be of normal, carburizing (acetylenic) and oxidizing structure.
Theoretically, the process of incomplete combustion of acetylene in pure oxygen can be characterized by the following equation: HCCH + O 2 → H 2 + CO + CO (one mole of O 2 is required for the reaction).
The resulting molecular hydrogen and carbon monoxide react with air oxygen. The final products are water and tetravalent carbon oxide. The equation looks like this: CO + CO + H 2 + 1½O 2 → CO 2 + CO 2 +H 2 O. This reaction requires 1.5 moles of oxygen. When summing up O 2, it turns out that 2.5 moles are spent per 1 mole of HCCH. And since in practice it is difficult to find ideally pure oxygen (often it has slight contamination impurities), then the ratio of O 2 to HCCH will be 1.10 to 1.20.
When the oxygen to acetylene ratio is less than 1.10, a carburizing flame occurs. Its structure has an enlarged core, its outlines become blurry. Soot is released from such a fire due to a lack of oxygen molecules.
If the gas ratio is greater than 1.20, then an oxidizing flame with an excess of oxygen is obtained. Its excess molecules destroy iron atoms and other components of the steel burner. In such a flame, the nuclear part becomes short and has points.
Temperature indicators
Each fire zone of a candle or burner has its own values, determined by the supply of oxygen molecules. The temperature of the open flame in its different parts ranges from 300 °C to 1600 °C.
An example is a diffusion and laminar flame, which is formed by three shells. Its cone consists of a dark area with a temperature of up to 360 °C and a lack of oxidizing substances. Above it is a glow zone. Its temperature ranges from 550 to 850 °C, which promotes thermal decomposition of the combustible mixture and its combustion.

The outer area is barely noticeable. In it, the flame temperature reaches 1560 °C, which is due to natural characteristics fuel molecules and the speed of entry of the oxidizing agent. This is where the combustion is most energetic.
Substances ignite at different temperature conditions. Thus, magnesium metal burns only at 2210 °C. For many solids flame temperature is about 350 °C. Matches and kerosene can ignite at 800 °C, while wood can ignite from 850 °C to 950 °C.
The cigarette burns with a flame whose temperature varies from 690 to 790 °C, and in a propane-butane mixture - from 790 °C to 1960 °C. Gasoline ignites at 1350 °C. The alcohol combustion flame has a temperature of no more than 900 °C.