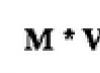What will heat up faster on the stove - a kettle or a bucket of water? The answer is obvious - a teapot. Then the second question is why?
The answer is no less obvious - because the mass of water in the kettle is less. Great. And now you can do a real physical experience yourself at home. To do this, you will need two identical small saucepans, an equal amount of water and vegetable oil, for example, half a liter each and a stove. Place saucepans with oil and water on the same heat. Now just watch what will heat up faster. If you have a thermometer for liquids, you can use it; if not, you can simply test the temperature with your finger from time to time, just be careful not to get burned. In any case, you will soon see that the oil heats up much faster than water. And one more question, which can also be implemented in the form of experience. What will boil faster - warm water or cold? Everything is obvious again - the warm one will be first at the finish line. Why all these strange questions and experiments? To determine the physical quantity called “amount of heat”.
Quantity of heat
The amount of heat is the energy that a body loses or gains during heat transfer. This is clear from the name. When cooling, the body will lose a certain amount of heat, and when heating, it will absorb. And the answers to our questions showed us What does the amount of heat depend on? Firstly, the greater the mass of a body, the greater the amount of heat that must be expended to change its temperature by one degree. Secondly, the amount of heat required to heat a body depends on the substance of which it consists, that is, on the type of substance. And thirdly, the difference in body temperature before and after heat transfer is also important for our calculations. Based on the above, we can determine the amount of heat using the formula:
where Q is the amount of heat,
m - body weight,
(t_2-t_1) - the difference between the initial and final body temperatures,
c is the specific heat capacity of the substance, found from the corresponding tables.
Using this formula, you can calculate the amount of heat that is necessary to heat any body or that this body will release when cooling.
The amount of heat is measured in joules (1 J), like any type of energy. However, this value was introduced not so long ago, and people began measuring the amount of heat much earlier. And they used a unit that is widely used in our time - calorie (1 cal). 1 calorie is the amount of heat required to heat 1 gram of water by 1 degree Celsius. Guided by these data, those who like to count calories in the food they eat can, for fun, calculate how many liters of water can be boiled with the energy they consume with food during the day.
The process of transferring energy from one body to another without doing work is called heat exchange or heat transfer. Heat exchange occurs between bodies having different temperatures. When contact is established between bodies with different temperatures, part of the internal energy is transferred from a body with a higher temperature to a body with a lower temperature. The energy transferred to a body as a result of heat exchange is called amount of heat.
Specific heat capacity of a substance:
If the heat transfer process is not accompanied by work, then, based on the first law of thermodynamics, the amount of heat is equal to the change in the internal energy of the body: .
The average energy of the random translational motion of molecules is proportional to the absolute temperature. The change in the internal energy of a body is equal to the algebraic sum of the changes in the energy of all atoms or molecules, the number of which is proportional to the mass of the body, therefore the change in internal energy and, therefore, the amount of heat is proportional to the mass and the change in temperature:


The proportionality factor in this equation is called specific heat capacity of a substance. Specific heat capacity shows how much heat is needed to heat 1 kg of a substance by 1 K.
Work in thermodynamics:
In mechanics, work is defined as the product of the moduli of force and displacement and the cosine of the angle between them. Work is done when a force acts on a moving body and is equal to the change in its kinetic energy.
In thermodynamics, the movement of a body as a whole is not considered; we are talking about the movement of parts of a macroscopic body relative to each other. As a result, the volume of the body changes, but its speed remains equal to zero. Work in thermodynamics is defined in the same way as in mechanics, but is equal to the change not in the kinetic energy of the body, but in its internal energy.
When work is performed (compression or expansion), the internal energy of the gas changes. The reason for this is: during elastic collisions of gas molecules with a moving piston, their kinetic energy changes.
Let us calculate the work done by the gas during expansion. The gas exerts a force on the piston  , Where
, Where  - gas pressure, and
- gas pressure, and  - surface area
- surface area  piston When gas expands, the piston moves in the direction of the force
piston When gas expands, the piston moves in the direction of the force  short distance
short distance  . If the distance is small, then the gas pressure can be considered constant. The work done by the gas is:
. If the distance is small, then the gas pressure can be considered constant. The work done by the gas is:
Where  - change in gas volume.
- change in gas volume.
In the process of gas expansion, it does positive work, since the direction of the force and displacement coincide. During the expansion process, the gas releases energy to surrounding bodies.
The work done by external bodies on a gas differs from the work done by a gas only in sign  , since the strength
, since the strength  , acting on the gas, is opposite to the force
, acting on the gas, is opposite to the force  , with which the gas acts on the piston, and is equal to it in modulus (Newton’s third law); but the movement remains the same. Therefore, the work of external forces is equal to:
, with which the gas acts on the piston, and is equal to it in modulus (Newton’s third law); but the movement remains the same. Therefore, the work of external forces is equal to:
 .
.
First law of thermodynamics:
The first law of thermodynamics is the law of conservation of energy, extended to thermal phenomena. Law of energy conservation: Energy in nature does not arise from nothing and does not disappear: the amount of energy is unchanged, it only passes from one form to another.
Thermodynamics considers bodies whose center of gravity remains virtually unchanged. The mechanical energy of such bodies remains constant, and only the internal energy can change.
Internal energy can change in two ways: heat transfer and work. In the general case, internal energy changes both due to heat transfer and due to work done. The first law of thermodynamics is formulated precisely for such general cases:
The change in the internal energy of a system during its transition from one state to another is equal to the sum of the work of external forces and the amount of heat transferred to the system:

If the system is isolated, then no work is done on it and it does not exchange heat with surrounding bodies. According to the first law of thermodynamics the internal energy of an isolated system remains unchanged.
Considering that  , the first law of thermodynamics can be written as follows:
, the first law of thermodynamics can be written as follows:

The amount of heat transferred to the system goes to change its internal energy and to perform work on external bodies by the system.
Second law of thermodynamics: It is impossible to transfer heat from a colder system to a hotter one in the absence of other simultaneous changes in both systems or in surrounding bodies.
In practice, thermal calculations are often used. For example, when constructing buildings, it is necessary to take into account how much heat the entire heating system should give to the building. You should also know how much heat will escape into the surrounding space through windows, walls, and doors.
We will show with examples how to carry out simple calculations.
So, you need to find out how much heat the copper part received when heated. Its mass was 2 kg, and the temperature increased from 20 to 280 °C. First, using Table 1, we determine the specific heat capacity of copper with m = 400 J / kg °C). This means that heating a copper part weighing 1 kg by 1 °C will require 400 J. To heat a copper part weighing 2 kg by 1 °C, the amount of heat required is 2 times greater - 800 J. The temperature of the copper part must be increased by more than 1 °C, and at 260 °C, this means that 260 times more heat will be required, i.e. 800 J 260 = 208,000 J.
If we denote the mass as m, the difference between the final (t 2) and initial (t 1) temperatures - t 2 - t 1, we obtain a formula for calculating the amount of heat:
Q = cm(t 2 - t 1).
Example 1. An iron cauldron weighing 5 kg is filled with water weighing 10 kg. How much heat must be transferred to the boiler with water to change its temperature from 10 to 100 °C?
When solving the problem, you need to take into account that both bodies - the boiler and the water - will heat up together. Heat exchange occurs between them. Their temperatures can be considered the same, i.e. the temperature of the boiler and water changes by 100 °C - 10 °C = 90 °C. But the amounts of heat received by the boiler and water will not be the same. After all, their masses and specific heat capacities are different.
Heating water in a pot
Example 2. We mixed water weighing 0.8 kg at a temperature of 25 °C and water at a temperature of 100 °C weighing 0.2 kg. The temperature of the resulting mixture was measured, and it turned out to be 40 °C. Calculate how much heat the hot water gave up when cooling and the cold water received when heated. Compare these amounts of heat.
Let's write down the conditions of the problem and solve it.


We see that the amount of heat given off by hot water and the amount of heat received by cold water are equal. This is not a random result. Experience shows that if heat exchange occurs between bodies, then the internal energy of all heating bodies increases by as much as the internal energy of cooling bodies decreases.
When conducting experiments, it usually turns out that the energy given off by hot water is greater than the energy received by cold water. This is explained by the fact that part of the energy is transferred to the surrounding air, and part of the energy is transferred to the vessel in which the water was mixed. The equality of energy given and received will be more accurate, the less energy loss is allowed in the experiment. If you calculate and take into account these losses, the equality will be exact.
Questions
- What do you need to know to calculate the amount of heat received by a body when heated?
- Explain with an example how the amount of heat imparted to a body when it is heated or released when it is cooled is calculated.
- Write a formula to calculate the amount of heat.
- What conclusion can be drawn from the experiment of mixing cold and hot water? Why are these energies not equal in practice?
Exercise 8
- How much heat is required to heat 0.1 kg of water by 1 °C?
- Calculate the amount of heat required to heat: a) a cast iron iron weighing 1.5 kg to change its temperature by 200 °C; b) an aluminum spoon weighing 50 g from 20 to 90 °C; c) a brick fireplace weighing 2 tons from 10 to 40 °C.
- How much heat was released when water with a volume of 20 liters cooled, if the temperature changed from 100 to 50 °C?
The focus of our article is the amount of heat. We will consider the concept of internal energy, which is transformed when this quantity changes. We will also show some examples of the use of calculations in human activity.
Heat
Every person has their own associations with any word in their native language. They are determined by personal experience and irrational feelings. What do you usually think of when you hear the word “warmth”? A soft blanket, a working central heating radiator in winter, the first sunlight in spring, a cat. Or a mother’s look, a friend’s comforting word, timely attention.
Physicists mean a very specific term by this. And very important, especially in some sections of this complex but fascinating science.

Thermodynamics
It is not worth considering the amount of heat in isolation from the simplest processes on which the law of conservation of energy is based - nothing will be clear. Therefore, first let us remind our readers of them.
Thermodynamics considers any thing or object as a combination of a very large number of elementary parts - atoms, ions, molecules. Its equations describe any change in the collective state of the system as a whole and as a part of the whole when macroparameters change. The latter refers to temperature (denoted as T), pressure (P), concentration of components (usually C).
Internal energy
Internal energy is a rather complex term, the meaning of which is worth understanding before talking about the amount of heat. It denotes the energy that changes when the value of the macroparameters of an object increases or decreases and does not depend on the reference system. It is part of the total energy. It coincides with it in conditions when the center of mass of the thing under study is at rest (that is, there is no kinetic component).
When a person feels that an object (say, a bicycle) has warmed up or cooled down, this indicates that all the molecules and atoms that make up that system have experienced a change in internal energy. However, the constant temperature does not mean the preservation of this indicator.
Work and heat

The internal energy of any thermodynamic system can be transformed in two ways:
- by doing work on it;
- during heat exchange with the environment.
The formula for this process looks like this:
dU=Q-A, where U is internal energy, Q is heat, A is work.
Let the reader not be deceived by the simplicity of the expression. The rearrangement shows that Q=dU+A, however, the introduction of entropy (S) brings the formula to the form dQ=dSxT.
Since in this case the equation takes the form of a differential one, the first expression requires the same. Next, depending on the forces acting in the object under study and the parameter that is being calculated, the required ratio is derived.
Let's take a metal ball as an example of a thermodynamic system. If you press on it, throw it up, drop it into a deep well, then this means doing work on it. Outwardly, all these harmless actions will not cause any harm to the ball, but its internal energy will change, albeit very slightly.
The second method is heat exchange. Now we come to the main goal of this article: a description of what the amount of heat is. This is a change in the internal energy of a thermodynamic system that occurs during heat exchange (see formula above). It is measured in joules or calories. Obviously, if you hold the ball over a lighter, in the sun, or simply in a warm hand, it will heat up. And then you can use the change in temperature to find the amount of heat that was communicated to him.
Why gas is the best example of a change in internal energy, and why schoolchildren don’t like physics because of this

Above we described changes in the thermodynamic parameters of a metal ball. They are not very noticeable without special devices, and the reader can only take the word about the processes occurring with the object. It's another matter if the system is gas. Press on it - it will be visible, heat it - the pressure will rise, lower it underground - and it can be easily recorded. Therefore, in textbooks, gas is most often used as a visual thermodynamic system.
But, alas, in modern education not much attention is paid to real experiences. The scientist who writes the methodological manual understands perfectly what is at stake. It seems to him that using the example of gas molecules, all thermodynamic parameters will be properly demonstrated. But a student who is just discovering this world is bored hearing about an ideal flask with a theoretical piston. If the school had real research laboratories and allocated hours to work in them, things would be different. So far, unfortunately, the experiments are only on paper. And, most likely, this is precisely the reason why people consider this branch of physics to be something purely theoretical, far from life and unnecessary.

Therefore, we decided to use the bicycle already mentioned above as an example. A person presses on the pedals and does work on them. In addition to imparting torque to the entire mechanism (thanks to which the bicycle moves in space), the internal energy of the materials from which the levers are made changes. The cyclist presses the handles to turn, and again does the work.
The internal energy of the outer coating (plastic or metal) increases. A person rides out into a clearing under the bright sun - the bicycle heats up, its amount of heat changes. Stops to rest in the shade of an old oak tree and the system cools, losing calories or joules. Increases speed - increases energy exchange. However, calculating the amount of heat in all these cases will show a very small, imperceptible value. Therefore, it seems that there are no manifestations of thermodynamic physics in real life.
Application of calculations for changes in the amount of heat
The reader will probably say that all this is very educational, but why are we so tormented at school with these formulas? And now we will give examples in which areas of human activity they are directly needed and how this concerns anyone in their everyday life.
First, look around you and count: how many metal objects surround you? Probably more than ten. But before becoming a paper clip, a carriage, a ring or a flash drive, any metal undergoes smelting. Each plant that processes, say, iron ore, must understand how much fuel is required in order to optimize costs. And when calculating this, it is necessary to know the heat capacity of the metal-containing raw material and the amount of heat that needs to be imparted to it in order for all technological processes to occur. Since the energy released by a unit of fuel is calculated in joules or calories, the formulas are needed directly.
Or another example: most supermarkets have a department with frozen goods - fish, meat, fruit. Where raw materials from animal meat or seafood are transformed into semi-finished products, they must know how much electricity refrigeration and freezing units will consume per ton or unit of finished product. To do this, you need to calculate how much heat a kilogram of strawberries or squid loses when cooled by one degree Celsius. And in the end, this will show how much electricity a freezer of a certain power will consume.
Planes, ships, trains

Above we showed examples of relatively motionless, static objects to which a certain amount of heat is imparted or from which, on the contrary, a certain amount of heat is taken away. For objects that move in conditions of constantly changing temperature during operation, calculations of the amount of heat are important for another reason.
There is such a thing as “metal fatigue”. It also includes maximum permissible loads at a certain rate of temperature change. Imagine an airplane taking off from the humid tropics into the frozen upper atmosphere. Engineers have to work hard to ensure that it does not fall apart due to cracks in the metal that appear when the temperature changes. They are looking for an alloy composition that can withstand real loads and have a large margin of safety. And in order not to search blindly, hoping to accidentally stumble upon the desired composition, you have to do a lot of calculations, including those that include changes in the amount of heat.