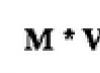The world around us, with all its richness and diversity, lives according to laws that are quite easy to explain with the help of sciences such as physics and chemistry. And even the basis of the life activity of such a complex organism as a person is nothing more than chemical phenomena and processes.
Definitions and examples
An elementary example is a kettle put on fire. After some time, the water will begin to heat up and then boil. We will hear a characteristic hissing sound, and streams of steam will fly out of the neck of the kettle. Where did it come from, because it wasn’t originally in the dishes! Yes, but water, at a certain temperature, begins to turn into gas, changing its physical state from liquid to gaseous. Those. it remained the same water, only now in the form of steam. This
And we will see chemical phenomena if we put a bag of tea leaves into boiling water. The water in a glass or other container will turn red-brown. A chemical reaction will occur: under the influence of heat, the tea leaves will begin to steam, releasing the color pigments and flavor properties inherent in this plant. We will get a new substance - a drink with specific qualitative characteristics characteristic only of it. If we add a few spoons of sugar there, it will dissolve (physical reaction), and the tea will become sweet. Thus, physical and chemical phenomena are often related and interdependent. For example, if the same tea bag is placed in cold water, no reaction will occur, the tea leaves and water will not interact, and the sugar will not want to dissolve either.
Thus, chemical phenomena are those in which some substances are converted into others (water into tea, water into syrup, firewood into ash, etc.) Otherwise, a chemical phenomenon is called a chemical reaction.
Physical phenomena are those in which the chemical composition of a substance remains the same, but the size of the body, shape, etc. changes. (a deformed spring, water frozen into ice, a tree branch broken in half).
Conditions of occurrence and occurrence
We can judge whether chemical and physical phenomena are occurring by certain signs and changes that are observed in a particular body or substance. Thus, most chemical reactions are accompanied by the following “identifying signs”:
- as a result or during its occurrence, a precipitate occurs;
- the color of the substance changes;
- Gases, such as carbon monoxide, may be released during combustion;
- heat is absorbed or, conversely, released;
- light emission is possible.
For chemical phenomena to be observed, i.e. reactions occur, certain conditions are necessary:
- the reacting substances must come into contact, be in contact with each other (i.e. the same tea leaves must be poured into a mug with boiling water);
- It is better to grind the substances, then the reaction will proceed faster, interaction will occur sooner (granulated sugar is more likely to dissolve and melt in hot water than lump sugar);
- In order for many reactions to occur, it is necessary to change the temperature regime of the reacting components, cooling or heating them to a certain temperature.
You can observe a chemical phenomenon experimentally. But you can describe it on paper using a chemical reaction).
Some of these conditions also work for the occurrence of physical phenomena, for example, a change in temperature or direct contact of objects and bodies with each other. For example, if you hit the head of a nail hard enough with a hammer, it can become deformed and lose its normal shape. But it will remain the head of a nail. Or, when you turn on the electric lamp, the tungsten filament inside it will begin to heat up and glow. However, the substance from which the thread is made will remain the same tungsten.
The description of physical processes and phenomena occurs through physical formulas and the solution of physical problems.
Key words of the abstract: Physical phenomena, chemical phenomena, chemical reactions, signs of chemical reactions, the meaning of physical and chemical phenomena.
Physical phenomena- these are phenomena in which usually only the state of aggregation of substances changes. Examples of physical phenomena are the melting of glass and the evaporation or freezing of water.
Chemical phenomena- these are phenomena as a result of which other substances are formed from given substances. In chemical phenomena, starting substances are transformed into other substances that have different properties. Examples of chemical phenomena are the combustion of fuel, the rotting of organic matter, the rusting of iron, and the souring of milk.
Chemical phenomena are also called chemical reactions.
Conditions for the occurrence of chemical reactions
The fact that during chemical reactions some substances are converted into others can be judged by external signs: release of heat (sometimes light), change in color, appearance of odor, formation of sediment, release of gas.
For many chemical reactions to begin, it is necessary to bring them into close contact of reacting substances . To do this, they are crushed and mixed; The contact area of the reacting substances increases. The finest crushing of substances occurs when they dissolve, so many reactions are carried out in solutions.
Grinding and mixing substances is only one of the conditions for the occurrence of a chemical reaction. For example. When sawdust comes into contact with air at normal temperatures, the sawdust does not ignite. In order for a chemical reaction to begin, in many cases it is necessary to heat substances to a certain temperature.

It is necessary to distinguish between concepts "conditions of occurrence" And “conditions for the flow of chemical reactions” . So, for example, in order for combustion to begin, heating is only necessary at the beginning, and then the reaction proceeds with the release of heat and light, and further heating is not required. And in the case of water decomposition, an influx of electrical energy is necessary not only to start the reaction, but also for its further course.
The most important conditions for the occurrence of chemical reactions are:
- thorough grinding and mixing of substances;
- preheating substances to a certain temperature.
The meaning of physical and chemical phenomena
Chemical reactions are of great importance. They are used to produce metals, plastics, mineral fertilizers, medicines, etc., and also serve as a source of various types of energy. Thus, when fuel burns, heat is released, which is used in everyday life and in industry.
All vital processes (respiration, digestion, photosynthesis, etc.) occurring in living organisms are also associated with various chemical transformations. For example, chemical transformations of substances contained in food (proteins, fats, carbohydrates) occur with the release of energy, which is used by the body to support vital processes.
Lesson summary “Physical and chemical phenomena (chemical reactions).”
>> Physical and chemical phenomena (chemical reactions). Let's experiment at home. External effects in chemical reactions
Physical and chemical phenomena (chemical reactions)
The material in this paragraph will help you figure out:
>what is the difference between physical and chemical phenomena.(chemical reactions);
> what external effects accompany chemical reactions.
In natural history lessons, you learned that various physical and chemical phenomena occur in nature.
Physical phenomena.
Each of you has repeatedly observed how ice melts, water boils or freezes. Ice, water and water vapor consist of the same molecules, so they are one substance (in different states of aggregation).
Phenomena in which a substance does not transform into another are called physical.
Physical phenomena include not only changes in substances, but also the glow of hot bodies, the passage of electric current in metals, the spread of the smell of substances in the air, the dissolution of fat in gasoline, and the attraction of iron to a magnet. Such phenomena are studied by the science of physics.
Chemical phenomena (chemical reactions).
One of the chemical phenomena is combustion. Let's consider the process of burning alcohol (Fig. 46). It occurs with the participation of oxygen contained in the air. When burned, alcohol seemingly turns into a gaseous state, just as water turns into steam when heated. But that's not true. If the gas obtained as a result of the combustion of alcohol is cooled, then part of it will condense into liquid, but not into alcohol, but into water. The rest of the gas will remain. With the help of additional experiment it can be proven that this residue is carbon dioxide.
Rice. 46. Burning alcohol
So the alcohol that burns and oxygen, which participates in the combustion process, are converted into water and carbon dioxide.
Phenomena in which some substances are transformed into others are called chemical phenomena or chemical reactions.
Substances that enter into a chemical reaction are called starting substances, or reagents, and those that are formed are called final substances, or reaction products.
The essence of the chemical reaction considered is conveyed by the following entry:
alcohol + oxygen -> water + carbon dioxide
starting materials final substances
(reagents) (reaction products)
The reactants and products of this reaction are made up of molecules. During combustion, a high temperature is created. Under these conditions, the molecules of the reagents disintegrate into atoms, which, when combined, form molecules of new substances - products. Therefore, all atoms are conserved during the reaction.
If the reactants are two ionic substances, then they exchange their ions. Other variants of interaction of substances are also known.
External effects accompanying chemical reactions.
By observing chemical reactions, you can record the following effects:
Change in color (Fig. 47, a);
gas release (Fig. 47, b);
formation or disappearance of sediment (Fig. 47, c);
appearance, disappearance or change in odor;
release or absorption of heat;
the appearance of a flame (Fig. 46), sometimes a glow.

Rice. 47. Some external effects during chemical reactions: a - appearance
coloring; b - gas release; c - appearance of sediment
Laboratory experiment No. 3
The appearance of color as a result of the reaction
Are solutions of soda ash and phenolphthalein colored?
Add 2 drops of phenolphthalein solution to a portion of soda solution I-2. What color appeared?
Laboratory experiment No. 4
Release of gas as a result of the reaction
Add a little chloride acid to the soda ash solution. What are you observing?
Laboratory experiment No. 5
The appearance of a precipitate as a result of the reaction
Add 1 ml of copper sulfate solution to the soda ash solution. What's happening?
The appearance of a flame is a sign of a chemical reaction, i.e. it indicates a chemical phenomenon. Other external effects can also be observed during physical events. Let's give a few examples.
Example 1. Silver powder obtained in a test tube as a result of a chemical reaction is gray in color. If you melt it and then cool the melt, you will get a piece of metal, but not gray, but white, with a characteristic shine.
Example 2. If you heat natural water, gas bubbles will begin to emerge from it long before boiling. This is dissolved air; its solubility in water decreases when heated.
Example 3. An unpleasant odor in the refrigerator disappears if granules of silica gel, one of the silicon compounds, are placed in it. Silica gel absorbs molecules of various substances without destroying them. Activated carbon works in a similar way in a gas mask.
Example 4 . When water turns into steam, heat is absorbed, and when water freezes, heat is released.
To determine what kind of transformation has occurred - physical or chemical, you should carefully observe it, as well as comprehensively examine the substances before and after the experiment.
Chemical reactions in nature, everyday life and their significance.
Chemical reactions occur constantly in nature. Substances dissolved in rivers, seas, and oceans interact with each other, some react with oxygen. Plants absorb carbon dioxide from the atmosphere, water and dissolved substances from the soil and process them into proteins, fats, glucose, starch, vitamins, other compounds, as well as oxygen.
This is interesting
As a result of photosynthesis, about 300 billion tons of carbon dioxide are absorbed from the atmosphere each year, 200 billion tons of oxygen are released, and 150 billion tons of organic substances are formed.
Reactions involving oxygen, which enters living organisms during respiration, are very important.
Many chemical reactions accompany us in everyday life. They occur during frying meat, vegetables, baking bread, souring milk, fermenting grape juice, bleaching fabrics, burning various types of fuel, hardening cement and alabaster, blackening silver jewelry over time, etc.
Chemical reactions form the basis of such technological processes as the extraction of metals from ores, the production of fertilizers, plastics, synthetic fibers, medicines, and other important substances. By burning fuel, people provide themselves with heat and electricity. Using chemical reactions, they neutralize toxic substances and process industrial and household waste.
The occurrence of some reactions leads to negative consequences. Rusting of iron shortens the life of various mechanisms, equipment, vehicles, and leads to large losses of this metal. Fires destroy housing, industrial and cultural facilities, and historical values. Most foods spoil due to their interaction with oxygen in the air; in this case, substances are formed that have an unpleasant odor, taste and are harmful to humans.
conclusions
Physical phenomena are the phenomena in which each substance is conserved.
Chemical phenomena, or chemical reactions, are the transformation of one substance into another. They can be accompanied by various external effects.
Many chemical reactions occur in the environment, in plants, animals and humans, and accompany us in everyday life.
?
100. Match:
1) dynamite explosion; a) physical phenomenon;
2) solidification of molten paraffin; b) chemical phenomenon.
3) food burning in a frying pan;
4) the formation of salt during the evaporation of sea water;
5) separation of a strongly shaken mixture of water and vegetable oil;
6) fading of dyed fabric in the sun;
7) passage of electric current in the metal;
101. What external effects are accompanied by such chemical transformations: a) burning of a match; b) rust formation; c) fermentation of grape juice.
102. Why do you think some food products (sugar, starch, vinegar, salt) can be stored indefinitely, while others (cheese, butter, milk) quickly spoil?
Experimenting at home
External effects in chemical reactions
1. Prepare small amounts of aqueous solutions of citric acid and baking soda. Pour portions of both solutions together into a separate glass. What's happening?
Add a few soda crystals to the remainder of the citric acid solution, and a few citric acid crystals to the remainder of the soda solution. What effects do you observe - the same or different?
2. Pour some water into three small glasses and add 1-2 drops of brilliant green alcohol solution, known as “zelenka,” to each. Add a few drops of ammonia to the first glass, and citric acid solution to the second. Has the color of the dye (green) in these glasses changed? If so, how exactly?
Write down the results of the experiments in a notebook and draw conclusions.
Popel P. P., Kryklya L. S., Chemistry: Pidruch. for 7th grade. zagalnosvit. navch. closing - K.: VC "Academy", 2008. - 136 p.: ill.
Lesson content lesson notes and supporting frame lesson presentation interactive technologies accelerator teaching methods Practice tests, testing online tasks and exercises homework workshops and trainings questions for class discussions Illustrations video and audio materials photographs, pictures, graphs, tables, diagrams, comics, parables, sayings, crosswords, anecdotes, jokes, quotes Add-ons abstracts cheat sheets tips for the curious articles (MAN) literature basic and additional dictionary of terms Improving textbooks and lessons correcting errors in the textbook, replacing outdated knowledge with new ones Only for teachers calendar plans training programs methodological recommendationsFor the last 200 years of humanity studied the properties of substances better than in the entire history of the development of chemistry. Naturally, the number of substances is also growing rapidly; this is due, first of all, to the development of various methods for obtaining substances. In everyday life we come across many substances. Among them are water, iron, aluminum, plastic, soda, salt and many others. Substances that exist in nature, such as oxygen and nitrogen contained in the air, substances dissolved in water and of natural origin, are called natural substances. Aluminum, zinc, acetone, lime, soap, aspirin, polyethylene and many other substances do not exist in nature. They are obtained in the laboratory and produced by industry. Artificial substances are not found in nature; they are created from natural substances. Some substances that exist in nature can also be obtained in a chemical laboratory. Thus, when potassium permanganate is heated, oxygen is released, and when chalk is heated, oxygen is released. carbon dioxide. Scientists have learned to turn graphite into diamond; they are growing crystals of ruby, sapphire and malachite. So, along with substances of natural origin, there are a huge number of artificially created substances that are not found in nature. Substances not found in nature are produced in various enterprises: factories, factories, combines, etc. In the context of depletion of the natural resources of our planet, chemists now face an important task: to develop and implement methods by which it is possible to artificially, in a laboratory or industrial production, obtain substances that are analogues of natural substances.
For example, reserves of fossil fuels in nature are running out.
There may come a time when oil and natural gas run out. Already, new types of fuel are being developed that would be just as efficient, but would not pollute the environment. Today, humanity has learned to artificially obtain various precious stones, for example, diamonds, emeralds, and beryls. State of matter Substances can exist in several states of aggregation, three of which are known to you: solid, liquid, gaseous. For example, water in nature exists in all three states of aggregation: At a temperature of –79°C this substance “freezes” and turns into a solid state of aggregation. The everyday (trivial) name for such a substance is “dry ice”. This name is given to this substance due to the fact that “dry ice” turns into carbon dioxide without melting, that is, without transitioning to a liquid state of aggregation, which is present, for example, in water. Thus, an important conclusion can be drawn. A substance, when transitioning from one state of aggregation to another, does not transform into other substances. The process of a certain change, transformation, is called a phenomenon.Physical phenomena. Physical properties of substances.
Phenomena in which substances change their state of aggregation, but do not transform into other substances, are called physical. Each individual substance has certain properties. The properties of substances may be different or similar to each other. Each substance is described using a set of physical and chemical properties. Let's take water as an example. Water freezes and turns into ice at a temperature of 0°C, and boils and turns into steam at a temperature of +100°C. These phenomena are considered physical, since water has not turned into other substances, only a change in the state of aggregation occurs. These freezing and boiling points are physical properties specific to water. Properties of substances that are determined by measurements or visually in the absence of transformation of some substances into others are called physical Evaporation of alcohol, like evaporation of water – physical phenomena, substances change their state of aggregation. After the experiment, you can be sure that alcohol evaporates faster than water - these are the physical properties of these substances. The main physical properties of substances include the following: state of aggregation, color, odor, solubility in water, density, boiling point, melting point, thermal conductivity, electrical conductivity. Physical properties such as color, smell, taste, crystal shape can be determined visually using the senses, and density, electrical conductivity, melting and boiling points are determined by measurement. Information about the physical properties of many substances is collected in specialized literature, for example, in reference books. The physical properties of a substance depend on its state of aggregation. For example, the densities of ice, water and water vapor are different. Gaseous oxygen is colorless, but liquid oxygen is blue. Knowledge of physical properties helps to “recognize” many substances. For example,- The only metal that is red in color. Only table salt has a salty taste. Iodine- An almost black solid that turns into a purple vapor when heated. In most cases, to identify a substance, you need to consider several of its properties. As an example, let us characterize the physical properties of water:- color – colorless (in small volumes)
- smell - no smell
- state of aggregation - liquid under normal conditions
- density – 1 g/ml,
- boiling point – +100°С
- melting point – 0°C
- thermal conductivity – low
- electrical conductivity – pure water does not conduct electricity
Crystalline and amorphous substances
When describing the physical properties of solids, it is customary to describe the structure of the substance. If you examine a sample of table salt under a magnifying glass, you will notice that the salt consists of many tiny crystals. In salt deposits you can also find very large crystals. Crystals are solids in the shape of regular polyhedra. Crystals can have different shapes and sizes. Crystals of certain substances, such as table salt salt – fragile and easy to break. There are crystals that are quite hard. For example, diamond is considered one of the hardest minerals. If you examine table salt crystals under a microscope, you will notice that they all have a similar structure. If we consider, for example, glass particles, they will all have a different structure - such substances are called amorphous. Amorphous substances include glass, starch, amber, and beeswax.Amorphous substances are substances that do not have a crystalline structure
Chemical phenomena. Chemical reaction. If during physical phenomena substances, as a rule, only change their state of aggregation, then during chemical phenomena the transformation of some substances into other substances occurs. Here are some simple examples: burning of a match is accompanied by charring of wood and the release of gaseous substances, that is, an irreversible transformation of wood into other substances occurs. Another example: Over time, bronze sculptures become covered with a green coating. The fact is that bronze contains copper. This metal slowly interacts with oxygen, carbon dioxide and air moisture, as a result of which new green substances are formed on the surface of the sculpture The process of interaction of substances with the formation of new substances is called a chemical reaction. Chemical reactions occur all around us. Chemical reactions also occur within ourselves. In our body, transformations of many substances continuously occur; substances react with each other, forming reaction products. Thus, in a chemical reaction there are always reacting substances and substances formed as a result of the reaction.- Chemical reaction– the process of interaction of substances, as a result of which new substances with new properties are formed
- Reagents- substances that enter into a chemical reaction
- Products– substances formed as a result of a chemical reaction
A chemical reaction is represented in general form by a reaction diagram REAGENTS -> PRODUCTS
Where reagents– starting materials taken to carry out the reaction; products– new substances formed as a result of a reaction. Any chemical phenomena (reactions) are accompanied by certain signs, with the help of which chemical phenomena can be distinguished from physical ones. Such signs include changes in the color of substances, the release of gas, the formation of sediment, the release of heat, and the emission of light. Many chemical reactions are accompanied by the release of energy in the form of heat and light. As a rule, such phenomena are accompanied by combustion reactions. In combustion reactions in air, substances react with oxygen contained in the air. For example, the metal magnesium flares up and burns in air with a bright, blinding flame. This is why magnesium flash was used to create photographs in the first half of the 20th century. In some cases, it is possible to release energy in the form of light, but without releasing heat. One type of Pacific plankton is capable of emitting a bright blue light, clearly visible in the dark. The release of energy in the form of light is the result of a chemical reaction that occurs in the organisms of this type of plankton. RESULT
- There are two large groups of substances: substances of natural and artificial origin.
- Under normal conditions, substances can exist in three states of aggregation
- Properties of substances that are determined by measurements or visually in the absence of transformation of some substances into others are called physical
- Crystals are solids in the shape of regular polyhedra.
- Amorphous substances are substances that do not have a crystalline structure
- Chemical phenomena - phenomena of transformation of one substance into another
- Reagents are substances that enter into a chemical reaction.
- Products are substances formed as a result of a chemical reaction
- Chemical reactions can be accompanied by the release of gas, sediment, heat, light; change in color of substances
- Combustion is a complex physicochemical process of converting starting substances into combustion products during a chemical reaction, accompanied by intense release of heat and light (flame)
Physical changes are not associated with chemical reactions and the creation of new products, such as melting ice. As a rule, such transformations are reversible. In addition to examples of physical phenomena, in nature and in everyday life there are also chemical transformations in which new products are formed. Such chemical phenomena (examples will be discussed in the article) are irreversible.
Chemical changes
Chemical changes can be thought of as any phenomenon that allows scientists to measure chemical properties. Many reactions are also examples of chemical phenomena. While it's not always easy to tell that a chemical change has occurred, there are some telltale signs. What are chemical phenomena? Let's give examples. This may be a change in the color of the substance, temperature, the formation of bubbles or (in liquids) the formation of a precipitate. The following examples of chemical phenomena in life can be given:
- Rust on iron.
- Wood burning.
- Metabolism of food in the body.
- Mixing acid and alkali.
- Cooking the egg.
- Digestion of sugar by amylase in saliva.
- Mixing baking soda and vinegar to create carbon dioxide gas.
- Baking a pie.
- Metal galvanization.
- Batteries.
- Fireworks explosion.
- Rotting bananas.
- Formation of lactic acid products.
And this is not the entire list. We can look at some of these points in more detail.

Outdoor fire using wood
Fire - This is also an example of a chemical phenomenon. This is the rapid oxidation of a material in an exothermic chemical combustion process, releasing heat, light and various reaction products. The fire is hot because there is a conversion of the weak double bond in molecular oxygen O 2 to the stronger bonds in the combustion products carbon dioxide and water. Great energy is released (418 kJ per 32 g O 2); The binding energies of the fuel play only a minor role here. At a certain point in the combustion reaction, called the flash point, flames are formed.
This is the visible part of fire and consists mainly of carbon dioxide, water vapor, oxygen and nitrogen. If the temperature is high enough, the gases can become ionized to produce plasma. Depending on what substances are ignited and what impurities are supplied from outside, the color of the flame and the intensity of the fire will be different. Fire in its most common form can result in a fire that can cause physical damage when burned. Fire is an important process that affects ecological systems around the world. The positive effects of fire include stimulating growth and maintaining various ecological systems.
Rust
Just like fire, the rusting process is also an oxidative process. Just not as fast-moving. Rust is an iron oxide, usually a red oxide, formed by the redox reaction of iron and oxygen in the presence of water or air. Several forms of rust are distinguished both visually and spectroscopically and form under different circumstances. Given enough time, oxygen and water, any mass of iron will eventually turn completely to rust and decompose. The surface portion is flaky and crumbly, and does not protect the underlying iron, unlike the patina that forms on copper surfaces.
An example of a chemical phenomenon, rusting is a general term for the corrosion of iron and its alloys such as steel. Many other metals undergo similar corrosion, but the resulting oxides are not usually called rust. Other forms of this reaction exist as a result of the reaction between iron and chloride in an oxygen-deprived environment. An example is the rebar used in underwater concrete pillars, which generates green rust.

Crystallization
Another example of a chemical phenomenon is crystal growth. It is a process in which a pre-existing crystal becomes larger as the number of molecules or ions at their positions in the crystal lattice increases. A crystal is defined as atoms, molecules or ions arranged in an ordered repeating pattern, a crystal lattice, extending in all three spatial dimensions. Thus, crystal growth differs from the growth of a liquid drop in that during growth, molecules or ions must fall into the correct lattice positions for an ordered crystal to grow.

When molecules or ions fall into positions different from those in an ideal crystal lattice, crystal defects are formed. Generally, molecules or ions in a crystal lattice are trapped in the sense that they cannot move from their positions, and therefore crystal growth is often irreversible, since once the molecules or ions have fallen into place in the growing lattice, they are fixed in it. Crystallization is a common process in both industry and the natural world, and crystallization is generally understood to consist of two processes. If there was no previously existing crystal, then a new crystal must be born, and then it must undergo growth.

Chemical origin of life
The chemical origin of life refers to the conditions that might have existed and therefore contributed to the emergence of the first duplicated life forms.

The main example of chemical phenomena in nature is life itself. It is believed that a combination of physical and chemical reactions could lead to the appearance of the first molecules, which, through reproduction, led to the emergence of life on the planet.