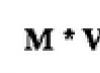; sometimes called "torr"(Russian designation - torr, international - Torr) in honor of Evangelista Torricelli.
The origin of this unit is associated with the method of measuring atmospheric pressure using a barometer, in which the pressure is balanced by a column of liquid. It is often used as a liquid because it has a very high density (≈13,600 kg/m³) and low saturated vapor pressure at room temperature.
Atmospheric pressure at sea level is approximately 760 mmHg. Art. Standard Atmosphere pressure taken equal to (exactly) 760 mm Hg. Art. , or 101,325 Pa, hence the definition of a millimeter mercury
(101,325/760 Pa). Previously, a slightly different definition was used: the pressure of a column of mercury with a height of 1 mm and a density of 13.5951·10 3 kg/m³ with a free fall acceleration of 9.806 65 m/s². The difference between these two definitions is 0.000014%.
Millimeters of mercury are used, for example, in vacuum technology, in weather reports and in measuring blood pressure. Since in vacuum technology very often pressure is measured simply in millimeters, omitting the words “mercury column”, the natural transition for vacuum engineers to microns (microns) is carried out, as a rule, also without indicating “mercury column pressure”. Accordingly, when a pressure of 25 microns is indicated on a vacuum pump, we are talking about the maximum vacuum created by this pump, measured in microns of mercury. Of course, no one uses a Torricelli pressure gauge to measure such low pressures. To measure low pressures, other instruments are used, for example, McLeod pressure gauge (vacuum gauge). 1 Sometimes millimeters of water column are used ( 13,5951 mmHg Art. = mm water Art. ). In the USA and Canada, the unit of measurement “inch of mercury” (symbol - inHg) is also used. 1 = 3,386389 inHg
| Pressure units Pascal |
(Pa, Pa) Bar |
(bar, bar) Technical atmosphere |
(at, at) Physical atmosphere |
(atm, atm) Millimeter of mercury |
(mm Hg, mm Hg, Torr, torr) Water column meter |
(m water column, m H 2 O) Pound-force per sq. inch |
|
|---|---|---|---|---|---|---|---|
| (psi) | 1 / 2 | 10 −5 | 1 Pa | 10.197 10 −6 | 9.8692 10 −6 | 7.5006 10 −3 | 1.0197 10 −4 |
| 145.04 10 −6 | 10 5 | 1 bar | 1,0197 | 0,98692 | 750,06 | 10,197 | 14,504 |
| 1 10 6 din/cm 2 | 98066,5 | 0,980665 | 1 at | 0,96784 | 735,56 | 10 | 14,223 |
| 1 kgf/cm 2 | 101325 | 1,01325 | 1,033 | 1 kgf/cm 2 | 760 | 10,33 | 14,696 |
| 1 atm | 133,322 | 1 mmHg Art. | 1.3332·10 −3 | 1.3595 10 −3 | 1 1.3158 10 −3 | mmHg Art. | 13.595 10 −3 |
| 1 m water Art. | 9806,65 | 9.80665 10 −2 | 0,1 | 0,096784 | 73,556 | 1 m water Art. | 1,4223 |
| 1 psi | 6894,76 | 68.948 10 −3 | 70.307 10 −3 | 68.046 10 −3 | 51,715 | 0,70307 | 1 lbf/in 2 |
see also
Write a review about the article "Millimeter of mercury"
Notes
Excerpt characterizing Millimeter of mercury
In October 1805, Russian troops occupied the villages and towns of the Archduchy of Austria, and more new regiments came from Russia and, burdening the residents with billeting, were stationed at the Braunau fortress. The main apartment of Commander-in-Chief Kutuzov was in Braunau.On October 11, 1805, one of the infantry regiments that had just arrived at Braunau, awaiting inspection by the commander-in-chief, stood half a mile from the city. Despite the non-Russian terrain and situation ( orchards, stone fences, tiled roofs, mountains visible in the distance), to the non-Russian people, looking at the soldiers with curiosity, the regiment had exactly the same appearance as any Russian regiment had, preparing for a review somewhere in the middle of Russia.
In the evening, on the last march, an order was received that the commander-in-chief would inspect the regiment on the march. Although the words of the order seemed unclear to the regimental commander, and the question arose how to understand the words of the order: in marching uniform or not? In the council of battalion commanders, it was decided to present the regiment in full dress uniform on the grounds that it is always better to bow than not to bow. And the soldiers, after a thirty-mile march, did not sleep a wink, they repaired and cleaned themselves all night; adjutants and company commanders counted and expelled; and by morning the regiment, instead of the sprawling, disorderly crowd that it had been the day before during the last march, represented an orderly mass of 2,000 people, each of whom knew his place, his job, and of whom, on each of them, every button and strap was in its place and sparkled with cleanliness . Not only was the outside in good order, but if the commander-in-chief had wanted to look under the uniforms, he would have seen an equally clean shirt on each one and in each knapsack he would have found the legal number of things, “sweat and soap,” as the soldiers say. There was only one circumstance about which no one could be calm. It was shoes. More than half the people's boots were broken. But this deficiency was not due to the fault of the regimental commander, since, despite repeated demands, the goods were not released to him from the Austrian department, and the regiment traveled a thousand miles.
The regimental commander was an elderly, sanguine general with graying eyebrows and sideburns, thick-set and wider from chest to back than from one shoulder to the other. He was wearing a new, brand new uniform with wrinkled folds and thick golden epaulettes, which seemed to lift his fat shoulders upward rather than downwards. The regimental commander had the appearance of a man happily performing one of the most solemn affairs of life. He walked in front of the front and, as he walked, trembled at every step, slightly arching his back. It was clear that the regimental commander was admiring his regiment, happy with it, that all his mental strength was occupied only with the regiment; but, despite the fact that his trembling gait seemed to say that, in addition to military interests, the interests of social life and the female sex occupied a significant place in his soul.
“Well, Father Mikhailo Mitrich,” he turned to one battalion commander (the battalion commander leaned forward smiling; it was clear that they were happy), “it was a lot of trouble this night.” However, it seems that nothing is wrong, the regiment is not bad... Eh?
Every person knows that air pressure is measured in millimeters of mercury, since this is the unit of measurement that is used in everyday life. In physics, in the SI system of units, pressure is measured in pascals. The article will tell you how to convert millimeters of mercury into pascals.
Air pressure
First, let's look at the question of what air pressure is. This value is understood as the pressure that the atmosphere of our planet exerts on any objects located on the surface of the Earth. It is easy to understand the reason for the appearance of this pressure: for this you need to remember that each body of finite mass has a certain weight, which can be determined by the formula: N = m*g, where N is the weight of the body, g is the value of the acceleration due to gravity, m is the mass of the body . The presence of weight in the body is due to gravity.
The atmosphere of our planet is a large gaseous body that also has some mass and therefore has weight. It has been experimentally established that the mass of air that exerts pressure on 1 m 2 of the earth's surface at sea level is approximately equal to 10 tons! The pressure exerted by this air mass is 101,325 pascals (Pa).
Converting millimeters of mercury to pascals
When viewing a weather forecast, barometric pressure information is usually presented in millimeters of mercury (mmHg). To understand how mmHg. Art. convert to pascals, you just need to know the relationship between these units. And remembering this ratio is easy: 760 mm Hg. Art. corresponds to a pressure of 101,325 Pa.
Knowing the above numbers, you can obtain a formula for converting millimeters of mercury into pascals. The easiest way to do this is to use a simple proportion. For example, a certain pressure H in mm Hg is known. Art., then the pressure P in pascals will be equal to: P = H*101325/760 = 133.322*H.

The given formula is easy to use. For example, at the top of Mount Elbrus (5642 m) the air pressure is approximately 368 mm Hg. Art. Substituting this value into the formula, we get: P = 133.322*H = 133.322*368 = 49062 Pa, or approximately 49 kPa.
In which the pressure is balanced by a column of liquid. It is often used as a liquid because it has a very high density (≈13,600 kg/m³) and low saturated vapor pressure at room temperature.
Atmospheric pressure at sea level is approximately 760 mmHg. Art.
(101,325/760 Pa). Previously, a slightly different definition was used: the pressure of a column of mercury with a height of 1 mm and a density of 13.5951·10 3 kg/m³ with a free fall acceleration of 9.806 65 m/s². The difference between these two definitions is 0.000014%.
Millimeters of mercury are used, for example, in vacuum technology, in weather reports and in measuring blood pressure. Since in vacuum technology very often pressure is measured simply in millimeters, omitting the words “mercury column”, the natural transition for vacuum engineers to microns (microns) is carried out, as a rule, also without indicating “mercury column pressure”. Accordingly, when a pressure of 25 microns is indicated on a vacuum pump, we are talking about the maximum vacuum created by this pump, measured in microns of mercury. Of course, no one uses a Torricelli pressure gauge to measure such low pressures. To measure low pressures, other instruments are used, for example, McLeod pressure gauge (vacuum gauge). 1 Sometimes millimeters of water column are used ( 13,5951 mmHg Art. = Standard atmospheric pressure is taken to be (exactly) 760 mmHg. Art. , or 101,325 Pa, hence the definition of a millimeter of mercury (101,325/760 Pa). Previously, a slightly different definition was used: the pressure of a column of mercury with a height of 1 mm and a density of 13.5951·10 3 kg/m³ with a free fall acceleration of 9.806 65 m/s². The difference between these two definitions is 0.000014%. ). In the USA and Canada, the unit of measurement “inch of mercury” (symbol - inHg) is also used. 1 = 3,386389 inHg
| ). In the USA and Canada, the unit of measurement “inch of mercury” (designation - inHg) is also used. 1 Pascal |
(Pa, Pa) Bar |
(bar, bar) Technical atmosphere |
(at, at) Physical atmosphere |
(atm, atm) (atm, atm) |
(mm Hg, mmHg, Torr, torr) Water column meter |
(m water column, m H 2 O) Pound-force per sq. inch |
|
|---|---|---|---|---|---|---|---|
| (psi) | 1 / 2 | 10 −5 | 1 Pa | 10.197 10 −6 | 1 Pa | 7.5006 10 −3 | 1.0197 10 −4 |
| 7.5006 10 −3 | 10 5 | 1 bar | 1,0197 | 0,98692 | 750,06 | 10,197 | 14,504 |
| 1 bar | 98066,5 | 0,980665 | 1 at | 0,96784 | 735,56 | 10 | 14,223 |
| 1 at | 101325 | 1,01325 | 1,033 | 1 at | 760 | 10,33 | 14,696 |
| 1 atm | 133,322 | 1 mmHg Art. | 1.3332·10 −3 | 1.3595 10 −3 | 1 1 mmHg | mmHg Art. | 13.595 10 −3 |
| mmHg. | 9806,65 | 9.80665 10 −2 | 0,1 | 0,096784 | 73,556 | 1 m water Art. | 1,4223 |
| 1 m water Art. | 6894,76 | 68.948 10 −3 | 70.307 10 −3 | 68.046 10 −3 | 51,715 | 0,70307 | 1 lbf/in 2 |
see also
1 psi
- Wikimedia Foundation.
- 2010.
Rodchenko, Alexander Mikhailovich
Shaikhet, Arkady Samoilovich See what “Millimeter of mercury” is in other dictionaries:
Non-system units pressure, app. when measuring atm. water vapor pressure, high vacuum, etc. Designation: Russian. - mmHg art., int. — mm Hg. 1 mmHg Art. equal to hydrostatic pressure of a column of mercury with a height of 1 mm and a density of 13.5951... ... Technical Translator's Guide
- – non-system units. pressure; 1 mmHg art. = 133.332 Pa = 1.35952 10 3 kgf/cm2 = 13.595 mm water. Art. [Physical encyclopedia. In 5 volumes. M.: Soviet Encyclopedia. Editor-in-chief A. M. Prokhorov. 1988.] Term heading: General terms... ... Encyclopedia of terms, definitions and explanations of building materials
Off-system unit of pressure; designation: mmHg Art. 1 mmHg Art. = 133.322 Pa = 13.5951 mm water column. * * * MILLIMETER OF MERCURY COLUMN MILLIMETER OF MERCURY, non-systemic unit of pressure; designation: mmHg Art. 1 mmHg Art. = 133.322... encyclopedic Dictionary
Torr, an off-system unit of pressure used when measuring atmospheric pressure of water vapor, high vacuum, etc. Designation: Russian mm Hg. Art., international mm Hg. 1 mm of mercury is equal to hydrostatic... Encyclopedic Dictionary of Metallurgy
- (mmHg) unit of pressure, as a result of which mercury in the column rises by 1 millimeter. 1 mmHg Art. = 133.3224 Pa... Dictionary in medicine
Torr, a non-systemic unit of pressure used in atmospheric pressure measurements, partial pressure water vapor, high vacuum, etc. Designations: Russian mm Hg. Art., international mm Hg. 1 mmHg cm equals... ... Great Soviet Encyclopedia
Non-system units not subject to use. pressure. Designation mm Hg. Art. 1 mmHg Art. = 133.322 Pa (see Pascal) ... Big Encyclopedic Polytechnic Dictionary
Off-system unit of pressure; designation: mmHg Art. 1 mmHg Art. = 133.322 Pa = 13.5951 mm water. st... Natural science. encyclopedic Dictionary
. The International Organization of Legal Metrology (OIML) in its recommendations classifies the millimeter of mercury as a unit of measurement “which may be used provisionally until a date fixed by national regulations, but which should not be introduced unless not in use.”
The origin of this unit is related to the method of measuring atmospheric pressure using a barometer, in which the pressure is balanced by a column of liquid. It is often used as a liquid because it has a very high density (≈13,600 kg/m³) and low saturated vapor pressure at room temperature.
Atmospheric pressure at sea level is approximately 760 mmHg. Art.
Standard atmospheric pressure is taken to be (exactly) 760 mmHg. Art. , or 101,325 Pa, hence the definition of a millimeter of mercury (101,325/760 Pa). Previously, a slightly different definition was used: the pressure of a column of mercury with a height of 1 mm and a density of 13.5951·10 3 kg/m³ with a free fall acceleration of 9.806 65 m/s². The difference between these two definitions is 0.000014%.
Millimeters of mercury are used, for example, in vacuum technology, in weather reports and in measuring blood pressure. Since in vacuum technology very often pressure is measured simply in millimeters, omitting the words “mercury column”, the natural transition for vacuum engineers to microns (microns) is carried out, as a rule, also without indicating “mercury column pressure”. Accordingly, when a pressure of 25 microns is indicated on a vacuum pump, we are talking about the maximum vacuum created by this pump, measured in microns of mercury. Of course, no one uses a Torricelli pressure gauge to measure such low pressures. To measure low pressures, other instruments are used, for example, McLeod pressure gauge (vacuum gauge). 1 Sometimes millimeters of water column are used ( 13,5951 mmHg Art. = mm water Art. ). In the USA and Canada, the unit of measurement “inch of mercury” (symbol - inHg) is also used. 1 = 3,386389 inHg
| ). In the USA and Canada, the unit of measurement “inch of mercury” (designation - inHg) is also used. 1 Pascal |
(Pa, Pa) Bar |
Millimeters of mercury are used, for example, in vacuum technology, in weather reports and in measuring blood pressure. Since in vacuum technology very often pressure is measured simply in millimeters, omitting the words “mercury column”, the natural transition for vacuum engineers to microns (microns) is carried out, as a rule, also without indicating “mercury column pressure”. Accordingly, when a pressure of 25 microns is indicated on a vacuum pump, we are talking about the maximum vacuum created by this pump, measured in microns of mercury. Of course, no one uses a Torricelli pressure gauge to measure such low pressures. To measure low pressures, other instruments are used, for example, McLeod pressure gauge (vacuum gauge). Technical atmosphere |
Technical atmosphere Physical atmosphere |
Millimeter of mercury |
Physical atmosphere Water column meter |
Meter of water column per sq. inch |
|
|---|---|---|---|---|---|---|---|
| (psi) | Pound-force per square inch | 10 −5 | 1/² | 10.197 10 −6 | 1 Pa | 9.8692 10 −6 | 1.0197 10 −4 |
| 7.5006 10 −3 | 10 5 | 145.04 10 −6 | 1,0197 | 0,98692 | 750,06 | 10,197 | 14,504 |
| 1 bar | 98066,5 | 0,980665 | 1·10 6 din/cm² | 0,96784 | 735,56 | 10 | 14,223 |
| 1 at | 101325 | 1,01325 | 1,033 | 1 at | 760 | 10,33 | 14,696 |
| 1 kgf/cm² | 133,322 | 1 mmHg Art. | 1.3332·10 −3 | 1.3595 10 −3 | 1 1.3158 10 −3 | mmHg | Art. |
| mmHg. | 9806,65 | 13.595 10 −3 | 0,1 | 0,096784 | 73,556 | 19.337 10 −3 | 1,4223 |
| 1 m water Art. | 6894,76 | 9.80665 10 −2 | 1 m aq. | Art. | 51,715 | 0,70307 | 68.948 10 −3 |
70.307 10 −3
1 / 3
68.046 10 −3
1 lbf/in²
Encyclopedic YouTube
More about pressure
Hello. In this episode of the TranslatorsCafe.com channel we will talk about pressure. First, we will look at the units used to measure it, and then we will discuss pressure in everyday life and technology, including pressure inside our body, and pressure during space flights. We'll also talk about the role of pressure in the formation of hydrocarbons and diamonds, and some fun experiments with pressure. Finally, we will look at how high pressure is used to make synthetic diamonds. In physics, pressure is defined as the force acting on a unit surface area. If two equal forces act on one larger and one smaller surface, then the pressure on the smaller surface will be greater. Agree, it is much worse if someone who wears stilettos steps on your foot than someone who wears sneakers.. Let's see this principle in action using a knife. Often, although not always, it is the relative pressure that is shown. Atmospheric pressure is the air pressure in a given location. It usually refers to the pressure of a column of air per unit surface area. Changes in atmospheric pressure affect weather and air temperature. People and animals sometimes suffer from severe pressure changes. Low blood pressure causes problems of varying severity in humans and animals, from mental and physical discomfort to fatal diseases. For this reason, aircraft cabins are maintained above atmospheric pressure at a given altitude because the atmospheric pressure at cruising altitude is too low. Atmospheric pressure decreases with altitude. People and animals living high in the mountains, such as the Himalayas, adapt to such conditions. Travelers, on the other hand, must take the necessary precautionary measures so as not to get sick due to the fact that the body is not used to this low pressure. Exacerbation of altitude sickness leads to serious complications such as acute mountain sickness, high altitude pulmonary edema, high altitude cerebral edema and extreme mountain sickness. The danger of altitude and mountain sickness begins at an altitude of 2400 meters above sea level. To avoid altitude sickness, doctors advise not to use depressants such as alcohol and sleeping pills, drink plenty of fluids, and rise to altitude gradually, for example, on foot rather than by transport. It's also good to eat plenty of carbohydrates and get plenty of rest, especially if you're going uphill quickly. These measures will allow the body to get used to the oxygen deficiency caused by low atmospheric pressure. If you follow these recommendations, your body will be able to produce more red blood cells to transport oxygen to the brain and internal organs. It is also possible that your heart rate and breathing rate may increase. First medical aid in such cases is provided immediately. It is important to move the patient to a lower altitude where the atmospheric pressure is higher, preferably to an altitude lower than 2400 meters above sea level. Medicines and portable hyperbaric chambers are also used. These are lightweight, portable chambers that can be pressurized using a foot pump. A patient with altitude sickness is placed in a chamber in which the pressure corresponding to a lower altitude is maintained. Such a chamber is used only for providing first aid, after which the patient must be lowered below. Pilots and astronauts have to work in low-pressure environments, so they wear spacesuits that compensate for the low pressure environment. Space suits completely protect a person or animal from the environment. They are used in space. Altitude compensation suits are used by pilots- this is the pressure in the arteries. It is represented by two values: systolic, or the highest pressure during compression of the heart muscle, and diastolic, or the lowest pressure during relaxation of the heart muscle. Devices for measuring blood pressure are called sphygmomanometers or tonometers. For a unit blood pressure millimeters of mercury are used. Even in America and England! The Pythagorean mug is an interesting vessel that uses hydrostatic pressure, and specifically the siphon principle. According to legend, Pythagoras invented this mug to control the amount of wine he drank. According to other sources, this cup was supposed to control the amount of water drunk during a drought. Inside the mug there is a curved U-shaped tube hidden under the dome. One end of the tube is longer and ends in a hole in the stem of the mug. The other, shorter end is connected by a hole to the inside bottom of the mug so that the water in the mug fills the tube. The principle of operation of a mug is similar to the operation of a toilet cistern. If the liquid level rises above the level of the tube, the liquid flows into the second half of the tube and flows out due to hydrostatic pressure. If the level, on the contrary, is lower, then you can safely use the mug. Pressure is an important concept in geology. Without pressure, the formation of gemstones, both natural and artificial, is impossible. this process. For example, diamonds are formed in the Earth's mantle, under conditions of high pressure and high temperature. During volcanic eruptions, magma moves diamonds to the upper layers of the Earth's surface. Some diamonds fall to Earth from meteorites, and scientists believe they formed on planets similar to Earth. The production of synthetic gemstones began in the 50s of the 20th century, and has been gaining popularity recently. Some buyers prefer natural gems, but artificial stones are becoming more and more popular due to the low price and lack of problems associated with mining natural gemstones. One of the technologies for growing diamonds in laboratory conditions is the method of growing crystals at high pressure and high temperature. IN special devices The carbon is heated to 1000 °C and subjected to pressure of about 5 gigapascals. Typically, a small diamond is used as the seed crystal, and graphite is used for the carbon base. From it a new diamond grows. This is the most common method of growing diamonds, especially as gemstones, due to its low cost. The properties of diamonds grown in this way are the same or better than those of natural stones. and high temperature is mainly used to synthesize diamonds, but recently this method has helped to improve natural diamonds or change their color. Various presses are used to artificially grow diamonds. The most expensive to maintain and the most complex of them is the cubic press. It is used primarily to enhance or change the color of natural diamonds. Diamonds grow in the press at a rate of approximately 0.5 carats per day.
Thank you for your attention. If you liked this video, please don't forget to subscribe to our channel! Length and distance converter Mass converter Volume converter bulk products and food products Area converter Volume and units converter in culinary recipes Temperature converter Pressure converter, mechanical stress , Young's modulus Energy and work converter Power converter Force converter Time converter Linear velocity converter Flat angle Thermal efficiency and fuel efficiency converter Number converter in various systems notation Converter of units of measurement of quantity of information Exchange rates Dimensions women's clothing and Shoe Sizes men's clothing and shoes Angular velocity and rotational speed converter Acceleration converter Angular acceleration converter Density converter Specific volume converter Moment of inertia converter Torque converter Torque converter Converter specific heat Combustion (by mass) Converter of energy density and specific heat of combustion of fuel (by volume) Converter of temperature difference Converter of coefficient of thermal expansion Converter of thermal resistance Converter of specific thermal conductivity Converter specific heat capacity Energy Exposure and Thermal Radiation Power Converter Density Converter heat flow Heat Transfer Coefficient Converter Volume Flow Converter Converter Molar flow rate converter Mass flow density converter Molar concentration converter Mass concentration in solution converter Dynamic (absolute) viscosity converter Kinematic viscosity converter Surface tension converter Vapor permeability converter Vapor permeability and vapor transfer rate converter Sound level converter Microphone sensitivity converter Sound pressure level (SPL) level converter sound pressure with the ability to select reference pressure Brightness converter Luminous intensity converter Illumination converter Resolution converter in computer graphics Frequency and wavelength converter Optical power in diopters and focal length Diopter Power and Lens Magnification (×) Electrical Charge Converter Linear Charge Density Converter Surface Charge Density Converter Volume Charge Density Converter electric current Linear current density converter Surface current density converter Electric field strength converter Electrostatic potential and voltage converter Converter electrical resistance Electrical resistivity converter Electrical conductivity converter Electrical conductivity converter Electrical capacitance Inductance converter American wire gauge converter Levels in dBm (dBm or dBmW), dBV (dBV), watts and other units Magnetomotive force converter Voltage converter magnetic field Magnetic flux converter Magnetic induction converter Radiation. Ionizing radiation absorbed dose rate converter Radioactivity. Radioactive decay converter Radiation. Exposure dose converter Radiation. Absorbed Dose Converter Decimal Prefix Converter Data Transfer Typography and Image Processing Units Converter Timber Volume Units Converter Calculation molar mass Periodic table chemical elements D. I. Mendeleev
1 pascal [Pa] = 0.00750063755419211 millimeter of mercury (0°C) [mmHg]
Initial value
Converted value
pascal exapascal petapascal terapascal gigapascal megapascal kilopascal hectopascal decapascal decipascal centipascal millipascal micropascal nanopascal picopascal femtopascal attopascal newton per square meter meter newton per square meter centimeter newton per square meter millimeter kilonewton per square meter meter bar millibar microbar dyne per sq. centimeter kilogram-force per square meter. meter kilogram-force per square meter centimeter kilogram-force per square meter. millimeter gram-force per square meter centimeter ton-force (kor.) per sq. ft ton-force (kor.) per sq. inch ton-force (long) per sq. ft ton-force (long) per sq. inch kilopound-force per sq. inch kilopound-force per sq. inch lbf per sq. ft lbf per sq. inch psi poundal per sq. foot torr centimeter of mercury (0°C) millimeter of mercury (0°C) inch of mercury (32°F) inch of mercury (60°F) centimeter of water. column (4°C) mm water. column (4°C) inch water. column (4°C) foot of water (4°C) inch of water (60°F) foot of water (60°F) technical atmosphere physical atmosphere decibar walls per square meter piezo barium (barium) Planck pressure meter sea water foot of sea water (at 15°C) meter of water. column (4°C)
68.046 10 −3
General information

In physics, pressure is defined as the force acting on a unit surface area. If two equal forces act on one larger and one smaller surface, then the pressure on the smaller surface will be greater. Agree, it is much worse if someone who wears stilettos steps on your foot than someone who wears sneakers. For example, if you press with a blade sharp knife for tomato or carrot, the vegetable will be cut in half. The surface area of the blade in contact with the vegetable is small, so the pressure is high enough to cut that vegetable. If you press with the same force on a tomato or carrot with a dull knife, then most likely the vegetable will not cut, since the surface area of the knife is now larger, which means the pressure is less.
In the SI system, pressure is measured in pascals, or newtons per square meter.
Relative pressure
Sometimes pressure is measured as the difference between absolute and atmospheric pressure. This pressure is called relative or gauge pressure and is what is measured, for example, when checking the pressure in car tires. Measuring instruments often, although not always, indicate relative pressure.
Atmosphere pressure
Atmospheric pressure is the air pressure at a given location. It usually refers to the pressure of a column of air per unit surface area. Changes in atmospheric pressure affect weather and air temperature. People and animals suffer from severe pressure changes. Low blood pressure causes problems of varying severity in humans and animals, from mental and physical discomfort to fatal diseases. For this reason, aircraft cabins are maintained above atmospheric pressure at a given altitude because the atmospheric pressure at cruising altitude is too low.

Atmospheric pressure decreases with altitude. People and animals living high in the mountains, such as the Himalayas, adapt to such conditions. Travelers, on the other hand, should take necessary measures precautions so as not to get sick due to the fact that the body is not accustomed to such low pressure. Climbers, for example, can suffer from altitude sickness, which is associated with a lack of oxygen in the blood and oxygen starvation of the body. This disease is especially dangerous if you stay in the mountains for a long time. Exacerbation of altitude sickness leads to serious complications such as acute mountain sickness, high altitude pulmonary edema, high altitude cerebral edema and extreme mountain sickness. The danger of altitude and mountain sickness begins at an altitude of 2400 meters above sea level. To avoid altitude sickness, doctors advise not to use depressants such as alcohol and sleeping pills, drink plenty of fluids, and rise to altitude gradually, for example, on foot rather than by transport. It's also good to eat a large number of carbohydrates, and rest well, especially if the uphill climb happened quickly. These measures will allow the body to get used to the oxygen deficiency caused by low atmospheric pressure. If you follow these recommendations, your body will be able to produce more red blood cells to transport oxygen to the brain and internal organs. To do this, the body will increase the pulse and breathing rate.
First medical aid in such cases is provided immediately. It is important to move the patient to a lower altitude where the atmospheric pressure is higher, preferably to an altitude lower than 2400 meters above sea level. Medicines and portable hyperbaric chambers are also used. These are lightweight, portable chambers that can be pressurized using a foot pump. A patient with altitude sickness is placed in a chamber in which the pressure corresponding to a lower altitude is maintained. This camera is used only for first aid medical care, after which the patient must be lowered lower.
Some athletes use low pressure to improve circulation. Typically, this requires training to take place under normal conditions, and these athletes sleep in a low-pressure environment. Thus, their body gets used to high altitude conditions and begins to produce more red blood cells, which, in turn, increases the amount of oxygen in the blood, and allows them to achieve better results in sports. For this purpose, special tents are produced, the pressure in which is regulated. Some athletes even change the pressure in the entire bedroom, but sealing the bedroom is an expensive process.
Spacesuits
Pilots and astronauts have to work in low pressure environments, so they wear pressure suits to compensate for the low pressure. environment. Space suits completely protect a person from the environment. They are used in space. Altitude-compensation suits are used by pilots at high altitudes - they help the pilot breathe and counteract low barometric pressure.
Hydrostatic pressure
Hydrostatic pressure is the pressure of a fluid caused by gravity. This phenomenon plays a huge role not only in technology and physics, but also in medicine. For example, blood pressure is the hydrostatic pressure of blood on the walls of blood vessels. Blood pressure is the pressure in the arteries. It is represented by two quantities: systolic, or the greatest pressure, and diastolic, or the lowest pressure during a heartbeat. Devices for measuring blood pressure are called sphygmomanometers or tonometers. The unit of blood pressure is millimeters of mercury.
The Pythagorean mug is an interesting vessel that uses hydrostatic pressure, and specifically the siphon principle. According to legend, Pythagoras invented this cup to control the amount of wine he drank. According to other sources, this cup was supposed to control the amount of water drunk during a drought. Inside the mug there is a curved U-shaped tube hidden under the dome. One end of the tube is longer and ends in a hole in the stem of the mug. The other, shorter end is connected by a hole to the inside bottom of the mug so that the water in the cup fills the tube. The principle of operation of the mug is similar to the operation of a modern toilet cistern. If the liquid level becomes higher than the level of the tube, the liquid flows into the second half of the tube and flows out, thanks to hydrostatic pressure. If the level, on the contrary, is lower, then you can safely use the mug.
Pressure in geology
Pressure is an important concept in geology. Without pressure, the formation of gemstones, both natural and artificial, is impossible. High pressure and high temperature are also necessary for the formation of oil from the remains of plants and animals. Unlike gems, which primarily form in rocks, oil forms at the bottom of rivers, lakes, or seas. Over time, more and more sand accumulates over these remains. The weight of water and sand presses on the remains of animal and plant organisms. Over time, this organic material sinks deeper and deeper into the earth, reaching several kilometers below the earth's surface. The temperature increases by 25 °C for every kilometer below the earth's surface, so at a depth of several kilometers the temperature reaches 50–80 °C. Depending on the temperature and temperature difference in the formation environment, natural gas may form instead of oil.
Natural gemstones
The formation of gemstones is not always the same, but pressure is one of the main components of this process. For example, diamonds are formed in the Earth's mantle, under conditions of high pressure and high temperature. During volcanic eruptions, diamonds move to the upper layers of the Earth's surface thanks to magma. Some diamonds fall to Earth from meteorites, and scientists believe they formed on planets similar to Earth.
Synthetic gemstones
The production of synthetic gemstones began in the 1950s and has been gaining popularity recently. Some buyers prefer natural gemstones, but artificial stones are becoming more and more popular due to the low price and lack of problems associated with the extraction of natural gemstones. Thus, many buyers choose synthetic gemstones because their extraction and sale is not associated with human rights violations, child labor and the financing of wars and armed conflicts.
One of the technologies for growing diamonds in laboratory conditions is the method of growing crystals at high pressure and high temperature. In special devices, carbon is heated to 1000 °C and subjected to pressure of about 5 gigapascals. Typically, a small diamond is used as the seed crystal, and graphite is used for the carbon base. From it a new diamond grows. This is the most common method of growing diamonds, especially as gemstones, due to its low cost. The properties of diamonds grown in this way are the same or better than those of natural stones. The quality of synthetic diamonds depends on the method used to grow them. Compared to natural diamonds, which are often clear, most man-made diamonds are colored.
Due to their hardness, diamonds are widely used in manufacturing. In addition, their high thermal conductivity, optical properties and resistance to alkalis and acids are valued. Cutting tools are often coated with diamond dust, which is also used in abrasives and materials. Most of the diamonds in production are artificial origin due to the low price and because the demand for such diamonds exceeds the ability to mine them in nature.
Some companies offer services for creating memorial diamonds from the ashes of the deceased. To do this, after cremation, the ashes are refined until carbon is obtained, and then a diamond is grown from it. Manufacturers advertise these diamonds as mementos of the departed, and their services are popular, especially in countries with large percentages of wealthy citizens, such as the United States and Japan.
Method of growing crystals at high pressure and high temperature
The method of growing crystals under high pressure and high temperature is mainly used to synthesize diamonds, but recently this method has been used to improve natural diamonds or change their color. Various presses are used to artificially grow diamonds. The most expensive to maintain and the most complex of them is the cubic press. It is used primarily to enhance or change the color of natural diamonds. Diamonds grow in the press at a rate of approximately 0.5 carats per day.
Do you find it difficult to translate units of measurement from one language to another? Colleagues are ready to help you. Post a question in TCTerms and within a few minutes you will receive an answer.